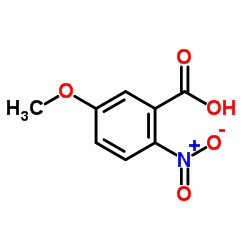
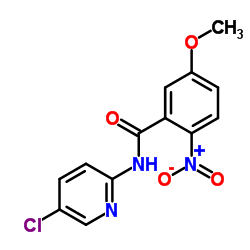
330942-05-7
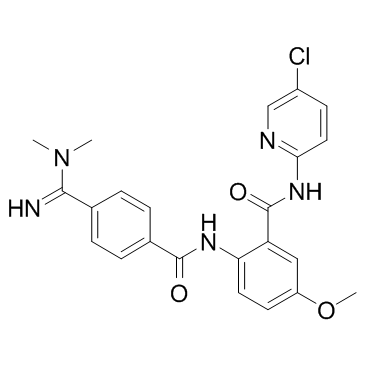
| Name | N-(5-chloropyridin-2-yl)-2-[[4-(N,N-dimethylcarbamimidoyl)benzoyl]amino]-5-methoxybenzamide |
|---|---|
| Synonyms |
N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide
UNII-74RWP7W0J9 QCR-99 N-(5-Chloro-2-pyridinyl)-2-{[4-(N,N-dimethylcarbamimidoyl)benzoyl]amino}-5-methoxybenzamide Betrixaban (USAN) N-(5-chloropyridin-2-yl)-2-{[4-(N,N-dimethylcarbamimidoyl)benzoyl]amino}-5-methoxybenzamide Betrixaban |
| Description | Betrixaban is a highly potent, selective, and orally efficacious factor Xa (fXa) inhibitor with IC50 of 1.5 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.5 nM (fXa)[1] Ki: 0.117 nM (fXa), 1.8 μM (hERG)[1] |
| In Vitro | In patch clamp hERG assays, Betrixaban has IC50 of 8.9 μM. The plasma kallikrein IC50 and Ki values for Betrixaban are 6.3 μM and 3.5 μM respectively. Betrixaban (hERG Ki 1.8 μM) exhibits significantly lower hERG activity than all the others (hERG Ki⩽0.5 μM)[1]. |
| In Vivo | Dosed at 0.5 mg/kg IV and 2.5 mg/kg PO, Betrixaban has bioavailability of 51.6% in dog; dosed at 0.75 mg/kg IV and 7.5 mg/kg PO, Betrixaban has bioavailability of 58.7% in monkey[1]. Both Betrixaban and Apixa-ban-mediated whole-blood INR increases are similarly reversed by r-Antidote. After i.v. infusion of the three fXa inhibitors (each admin¬istered individually) for 30 min, the total plasma concentrations of rivaroxaban, Betrixaban and apixaban are 1.4±0.4 μM (mean±s.d.), 0.2±0.01 μM and 1.4±0.3 μM, respectively, and the percentages of unbound inhibitor are 2.2%±0.8% (mean±s.d.), 40%±7.2% and 1.5%±0.3%, respectively. After administration of r-Antidote, the total plasma concentrations of the inhibitors increased to 1.9±0.09 μM, 2.0±0.4 μM and 4.2±0.7 μM, respectively, and the percentage of unbound inhibitor declined to 0%, 0.3%±0.1% and 0.05%±0.02%, respectively. Thus, for each of the three inhibitors, correction of prothrombin time by r-Antidote to near-normal values is associated with a reduction in the free fraction of the inhibitor[2]. |
| Kinase Assay | To measure the inhibition of fXa activity by direct fXa inhibitors and the reversal of its inhibitory effect by r-Antidote, purified human plasma fXa (3 nM) (Haematologic Technologies), varying concentrations of inhibitor (0, 2.5, 5.0 and 7.5 nM) and r-Antidote are added to the assay buffer (20 mM Tris, 150 mM NaCl, 5 mM Ca2+ and 0.1% BSA, pH 7.4). After incubation at room temperature for 30 min, 100 μM Spectrozyme-fXa is added to the mixture, and the initial rate of sub¬strate cleavage is monitored continuously for 5 min at 405 nm in a 96-well plate reader. The initial velocity of product formation as a function of inhibitor and r-Antidote concentrations is analyzed by Dynafit to estimate the binding affinity of r-Antidote to each inhibitor[2]. |
| Animal Admin | Rats[2] Whole-blood INR values (mean±s.d.) in rats infused with Betrixaban (1 mg/kg per hour) or vehicle and then treated with either vehicle or r-Antidote by i.v. bolus (6 mg) over 5 min plus infusion (9 mg/h) for up to 90 min. Circles, vehicle+vehicle; squares, Betrixaban + vehicle; triangles, Betrixaban + r-Antidote. *P≤0.02 compared to the r-Antidote treatment group determined by unpaired two-tailed t test. Whole-blood INR values (mean±s.d.) in rats infused with Apixaban (0.5 mg per kg body weight h−1) or vehicle and then treated with either vehicle or r-Antidote by i.v. bolus (6 mg) over 5 min plus infusion (6 mg/h) for up to 90 min. Circles, vehicle + vehicle; squares, apixaban + vehicle; triangles, apixaban+r-Antidote. *P≤0.01 compared to the r-Antidote treatment group determined by unpaired two-tailed t test. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C23H22ClN5O3 |
| Molecular Weight | 451.905 |
| Exact Mass | 451.141113 |
| PSA | 110.90000 |
| LogP | 2.93 |
| Appearance | light yellow solid |
| Index of Refraction | 1.629 |
| Storage condition | -20℃ |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
![N-(5-Chloro-2-pyridinyl)-2-[(4-cyanobenzoyl)amino]-5-methoxybenzamide structure](https://image.chemsrc.com/caspic/177/330942-01-3.png)