56684-87-8
| Name | 3-Methoxy-5-[2-(3-methoxyphenyl)ethyl]phenol |
|---|---|
| Synonyms |
3-Ethyl-1-<2-(3-indolyl)-ethyl>-piperidin
3-[2-(3-ethyl-piperidin-1-yl)-ethyl]-indole batatatsin III batatasin III 1-<2-(3-indolyl)ethyl>-3-ethylpiperidine 1H-Indole,3-[2-(3-ethyl-1-piperidinyl)ethyl] 3,3'-dihydroxy-5-methoxybibenzyl 3-[2-(3-hydroxyphenyl)ethyl]-5-methoxyphenol Batatasin Ⅲ |
| Description | Batatasin III, a stilbenoid, inhibits cancer migration and invasion by suppressing epithelial to mesenchymal transition (EMT) and FAK-AKT Signals. Batatasin III has anti-cancer activities[1]. |
|---|---|
| Related Catalog | |
| Target |
Akt |
| In Vitro | Batatasin III (25-100 μM; 48 h) exhibits anti-proliferative activity in H460 cells. Batatasin III at concentrations lower than 100 μM has no cytotoxic effects[1]. Batatasin III significantly suppresses EMT indicated by the decrease of N-cadherin and Vimentin, and up-regulation of E-cadherin[1]. |
| References |
| Density | 1.126g/cm3 |
|---|---|
| Boiling Point | 406.1ºC at 760 mmHg |
| Molecular Formula | C16H18O3 |
| Molecular Weight | 258.31200 |
| Flash Point | 199.4ºC |
| Exact Mass | 258.12600 |
| PSA | 38.69000 |
| LogP | 3.19460 |
| Index of Refraction | 1.572 |
|
~% 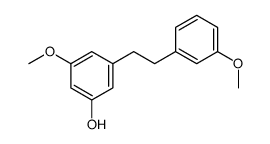
56684-87-8 |
| Literature: Hashimoto,T. et al. Phytochemistry (Elsevier), 1974 , vol. 13, p. 2849 - 2852 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |