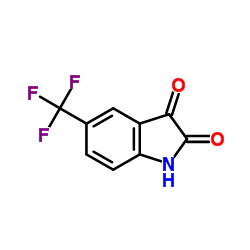
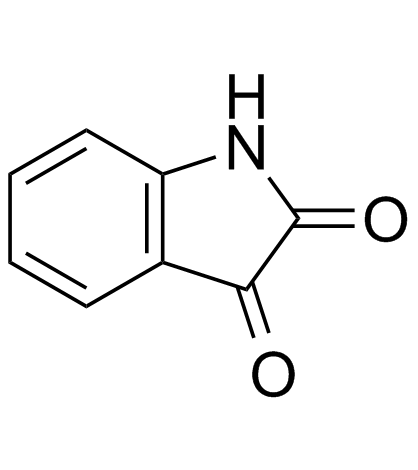
91-56-5
| Name | isatin |
|---|---|
| Synonyms |
Indolequinone
EINECS 202-077-8 MFCD00005718 indole-2,3-dione indoline-2,3-dione Indoquinone 2,3-Dioxoindoline 2,3-Dioxo-2,3-dihydroindole 2,3-Indolinedione 2,3-INDOLEDIONE 3-hydroxy-2-oxoindole ISATIC ANHYDRIDE Isotin 2,3-Diketoindoline 2-methopropan-1-ol 3-Hydroxy-2H-indol-2-one Isatin 2,3-KETOINDOLINE 5-21-10-00221 (Beilstein Handbook Reference) 2,3-dihydro-1H-indol-2,3-dione |
| Description | Isatin (Indoline-2,3-dione) is a potent inhibitor of monoamine oxidase (MAO) with an IC50 of 3 μM. Also binds to central benzodiazepine receptors (IC50 against clonazepam, 123 μM)[1]. Also acts as an antagonist of both atrial natriuretic peptide stimulated and nitric oxide-stimulated guanylate cyclase activity[2]. Shows effect on the serotonergic system[3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3 μM (MAO B)[1] |
| In Vitro | In dopaminergic SH-SY5Y cells isatin (1-400 μM) induces cell death in dose- and time dependent manner. This death occurred as a continuum of survival, apoptosis and necrosis[2]. |
| In Vivo | A single dose of isatin (80 mg/kg) has a rapid effect on the serotonergic system in the hypothalamus. Isatin significantly increases 5-HT concentrations in the hypothalamus and cortex but did not significantly alter 5-HIAA concentrations[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 360.3±52.0 °C at 760 mmHg |
| Melting Point | 193-195 °C (dec.)(lit.) |
| Molecular Formula | C8H5NO2 |
| Molecular Weight | 147.131 |
| Flash Point | 171.7±30.7 °C |
| Exact Mass | 147.032028 |
| PSA | 46.17000 |
| LogP | -0.17 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.679 |
| Storage condition | Store at RT. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi,Xn |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | 2811 |
| WGK Germany | 1 |
| RTECS | NL7873000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




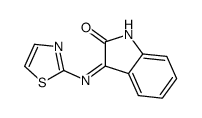
![3'-n-butyl-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione structure](https://image.chemsrc.com/caspic/205/1158178-15-4.png)
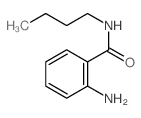
![methyl 2'-oxospiro[1,2,5,6,7,8-hexahydropyrrolizine-3,3'-1H-indole]-2-carboxylate structure](https://image.chemsrc.com/caspic/076/104680-77-5.png)
![2-[[[(2-oxoindol-3-yl)amino]carbamoylformyl]amino]benzoic acid structure](https://image.chemsrc.com/caspic/183/108097-98-9.png)