147536-97-8
| Name | bosentan |
|---|---|
| Synonyms |
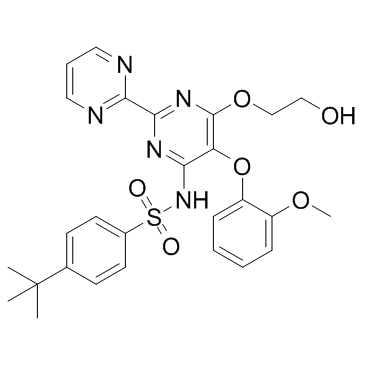
4-tert-Butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2,2'-bipyrimidin-4-yl]benzenesulfonamide
MFCD00867375 Bosentan Actelion ro47-0203 ro47-0203/039 4-(1,1-Dimethylethyl)-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)[2,2'-bipyrimidin]-4-yl]benzenesulfonamide N-[6-(2-Hydroxyethoxy)-5-(2-methoxyphenoxy)-2,2'-bipyrimidin-4-yl]-4-(2-methyl-2-propanyl)benzenesulfonamide UNII-XUL93R30K2 |
| Description | Bosentan is a competitive and dual antagonist of endothelin-1 (ET) for the ETA and ETB receptors with Ki of 4.7 nM and 95 nM in human SMC, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 4.7 nM (ETA receptor, in human SMC), 95 nM (ETA receptor, in human SMC)[1] |
| In Vitro | Bosentan (BOS) competitively and specifically antagonizes binding of 125I-labelled ET-1 to ETA receptors on human smooth muscle cells (SMC) and ETB receptors on human placenta cells. The in vitro binding affinity of Bosentan to ETA receptors on human SMC is 4.7 nM and to ETB receptors on human SMC or placenta cells is 41 or 95 nM. Bosentan has 67-fold greater selectivity for ETA than ETB receptors (mean IC50=7.1 vs 474.8 nM) in an in vitro 125I-labeling assay[1]. |
| In Vivo | Single-dose Bosentan 62.5 mg significantly (p<0.01 vs baseline) plasma ET-1 levels by 2-fold in 7 pts with WHO class II or III idiopathic or CTD-associated PAH, with peak levels achieved at 8 h[1]. In hypertensive rats, Macitentan 30 mg/kg further decreases mean arterial blood pressure (MAP) by 19 mm Hg when given on top of Bosentan 100 mg/kg. Conversely, Bosentan given on top of Macitentan fails to induce an additional MAP decrease. In pulmonary hypertensive rats, Macitentan 30 mg/kg further decreases mean pulmonary artery pressure (MPAP) by 4 mm Hg on top of Bosentan, whereas a maximal effective dose of Bosentan given on top of Macitentan does not cause any additional MPAP decrease[3]. |
| Cell Assay | Cell viability is evaluated by the trypan blue exclusion test. Human dermal fibroblasts are treated with the indicated concentration of Bosentan (10, 20 and 40 μM). Cell viability is examined at 24 and 48 hours. Stained (dead) and unstained (viable) cells are counted with a hematocytometer[2]. |
| Animal Admin | Rats[3] Two-month-old DSS rats and two-month-old Wistar rats are used. Pharmacological effects on mean arterial pressure (MAP) or mean pulmonary arterial pressure (MPAP) and heart rate (HR) are measured up to 120 h after a single gavage at doses ranging from 0.1 to 100 mg/kg (Macitentan) or 3 to 600 mg/kg (Bosentan). To determine whether Macitentan can provide superior pharmacological activity vs. Bosentan, a study is designed in which: 1) Macitentan is administered on top of the maximal effective dose of Bosentan established by the dose-response curve. 2) the same dose of Bosentan is administered on top of the maximal effective dose of Macitentan. The maximal effective dose of the second compound is administered at Tmax of the first tested compound. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 742.3±70.0 °C at 760 mmHg |
| Melting Point | 171-175 °C(lit.) |
| Molecular Formula | C27H29N5O6S |
| Molecular Weight | 551.614 |
| Flash Point | 402.8±35.7 °C |
| Exact Mass | 551.183838 |
| PSA | 154.03000 |
| LogP | 1.15 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.607 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36:Irritating to the eyes. R43:May cause sensitization by skin contact. |
| Safety Phrases | S26-S36/37-S24/25-S22 |
| WGK Germany | 3 |
| HS Code | 2935009090 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |