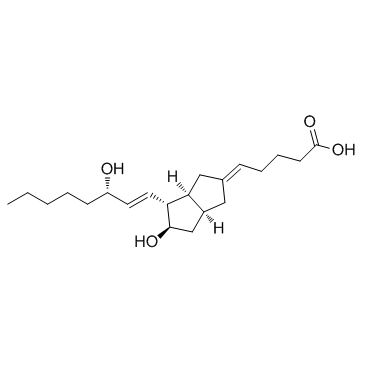
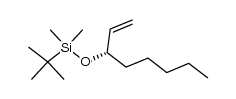
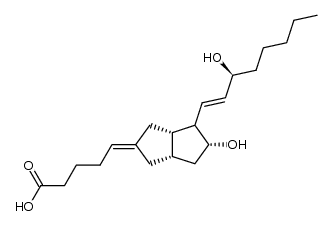
69552-46-1
| Name | (5E)-5-[(3aS,4R,5R,6aS)-5-hydroxy-4-[(E,3S)-3-hydroxyoct-1-enyl]-3,3a,4,5,6,6a-hexahydro-1H-pentalen-2-ylidene]pentanoic acid |
|---|---|
| Synonyms |
Carboprostacyclin
carbaprostacyclin carbacyclin 6a-Carba-pgi2 Carba pgx 6,9-Methano pgi2 6,9-Methanoprostaglandin I2 Carbacycline (5Z)-5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-[(1E,3S)-3-hydroxy-1-octen-1-yl]hexahydro-2(1H)-pentalenylidene]pentanoic acid (5Z)-5-[(3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydroxyoct-1-en-1-yl]hexahydropentalen-2(1H)-ylidene]pentanoic acid MFCD00210824 |
| Description | Carbacyclin is a PGI2 analogue, acts as a prostacyclin (PGI2) receptor agonist and vasodilator, and potently inhibits platelet aggregation. |
|---|---|
| Related Catalog | |
| Target |
PGI2 Receptor |
| In Vitro | Carbacyclin is an agonist of prostacyclin (PGI2) receptor[1]. Carbacyclin acts as an inhibitor of platelet aggregation induced by ADP or collagen in vitro[2]. Carbacyclin is a PGI2 analogue, activates CPT-1 mRNA expression through PPARδ, independent of the IP receptor signaling pathway. Carbacyclin (0.02 μM to 20 μM) activates the IP receptor signaling pathway via PKA, and such an effect is inhibited by H-89, a PKA inhibitor. Carbacyclin (0.02-80 μM) increases PPRE promoter activity via PPARδ independent of the IP receptor signaling pathway in cardiomyocytes[3]. |
| In Vivo | Carbacyclin is 0.03 times as active as prostacyclin on inhibiting platelet aggregation in human, dog or rabbit plasma[2]. Carbacyclin (100 μg, i.p.) induces CPT-1 mRNA expression in murine heart[3]. |
| Cell Assay | Primary cultures of neonatal rat cardiomyocytes are prepared from the ventricles of 1-day-old Wistar rats, and are seeded at a density of 4 × 105/6-well plastic plates, 9 × 105/60 mm dishes, or 3 × 106/100 mm dishes with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS). After 40 h of incubation, cultured cardiomyocytes are serum-starved for 8 h before Carbacyclin stimulation. |
| Animal Admin | Mice[3] Ten to twelve week-old male C57BL/6 mice (20-25 g) are used in the experiment. Mice (n = 4) are injected intraperitoneally with 100 μg of Carbacyclin, and are sacrificed at the times indicated. The hearts are excised, and the ventricles are then homogenized with 3 mL of Isogen for the following total RNA extraction procedure[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 514.8±50.0 °C at 760 mmHg |
| Melting Point | 65-67 °C |
| Molecular Formula | C21H34O4 |
| Molecular Weight | 350.492 |
| Flash Point | 279.2±26.6 °C |
| Exact Mass | 350.245697 |
| PSA | 77.76000 |
| LogP | 3.65 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.611 |
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | 22-36 |
| Precursor 10 | |
|---|---|
| DownStream 2 | |