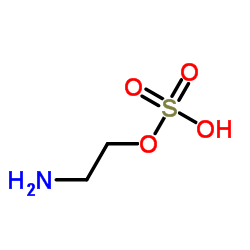
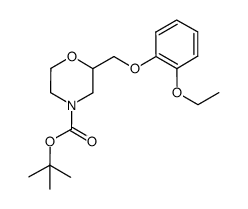
46817-91-8
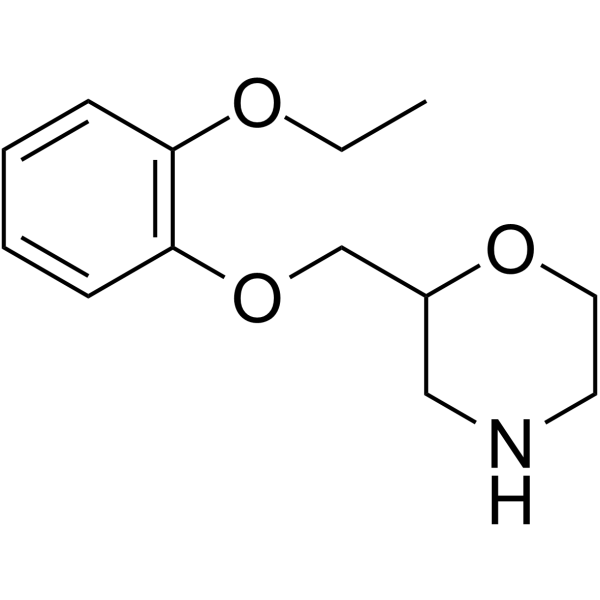
| Name | 2-[(2-ethoxyphenoxy)methyl]morpholine |
|---|---|
| Synonyms |
Emovit
Morpholine,2-((2-ethoxyphenoxy)methyl) Viloxazinum [INN-Latin] 2-(2-Ethoxy-phenoxymethyl)-morpholine 2-(o-ethoxyphenoxymethyl)morpholine viloxazine Viloxazina [INN-Spanish] Viloxazin |
| Description | Viloxazine (Viloxazin) is a norepinephrine reuptake inhibitor, also a potent 5-HT2C agonist and 5-HT2B antagonist with an EC50 of 32 μM and an IC50 of 27 μM for 5-HT2C and 5-HT2B, respectively. The mechanism of action of Viloxazine predominantly involves serotonergic and noradrenergic pathways. Viloxazine can be used for researching depression[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human 5-HT2C Receptor:32 μM (EC50) human 5-HT2B Receptor:27 μM (IC50) |
| References |
| Density | 1.061 g/cm3 |
|---|---|
| Boiling Point | 350.5ºC at 760 mmHg |
| Melting Point | 185-186ºC |
| Molecular Formula | C13H19NO3 |
| Molecular Weight | 237.29500 |
| Flash Point | 144.3ºC |
| Exact Mass | 237.13600 |
| PSA | 39.72000 |
| LogP | 1.78130 |
| Index of Refraction | 1.499 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 5 | |
|---|---|
| DownStream 0 | |