89778-26-7
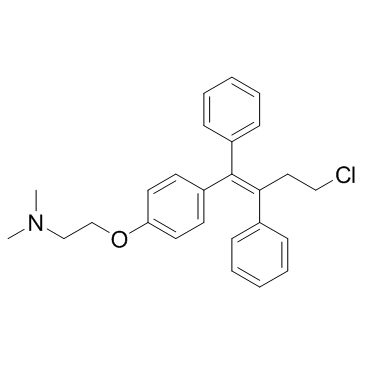
| Name | toremifene |
|---|---|
| Synonyms |
(Z)-Toremifene
2-{4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine MFCD00801070 2-{4-[(1Z)-4-Chloro-1,2-diphenyl-1-buten-1-yl]phenoxy}-N,N-dimethylethanamine 2-({4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenyl}oxy)-N,N-dimethylethanamine Toremifene Base 2-(4-(4-Chloro-1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-dimethylethanamine Toremifene 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine (Z)-4-Chloro-1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]phenyl]-1-butene (Z)-2-[4-(4-Chloro-1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine |
| Description | Toremifene (NK 622; FC 1157a) is a second-generation selective estrogen-receptor modulator (SERM) in development for the prevention of osteoporosis.IC50 Value: 1±0.3 μMTarget: Estrogen receptorToremifene is a second-generation selective estrogen-receptor modulator (SERM) in development for the prevention of osteoporosis and other adverse effects resulting from ADT in men with prostate cancer [1]. in vitro: The growth of Ac-1 cells was inhibited by tamoxifen, toremifene and atamestane in vitro with IC50values of 1.8±1.3μM, 1±0.3μM and 60.4±17.2μM, respectively. The combination of toremifene plusatamestane was found to be better than toremifene or atamestane alone in vitro[2].in vivo: The effect of this combination was then studied in vivo using Ac-1 xenografts grown in ovariectomized female SCID mice. The mice were injected with toremifene (1000μg/day), atamestane (1000μg/day), tamoxifen (100μg/day), or the combination of toremifene plus atamestane. In this study, our results indicate that the combination of toremifene plus atamestane was as effective as toremifene or tamoxifen alone but may not provide any additional benefit over toremifene alone or tamoxifen alone[2].Clinical trail: Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene[3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 535.1±50.0 °C at 760 mmHg |
| Melting Point | 108-110°C |
| Molecular Formula | C26H28ClNO |
| Molecular Weight | 405.960 |
| Flash Point | 277.4±30.1 °C |
| Exact Mass | 405.185944 |
| PSA | 12.47000 |
| LogP | 7.82 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.588 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R37:Irritating to the respiratory system. R43:May cause sensitization by skin contact. |
| Safety Phrases | S8-S24/25-S46 |
| RIDADR | UN 2210 |
| RTECS | ZB3200000 |
| Packaging Group | III |
| Hazard Class | 9 |
| HS Code | 2922299090 |
|
~% 
89778-26-7 |
| Literature: WO2011/13108 A1, ; Page/Page column 14 ; |
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
