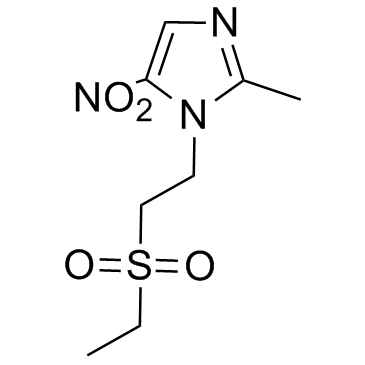
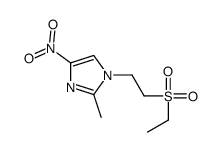
19387-91-8
| Name | 1-(2-ethylsulfonylethyl)-2-methyl-5-nitroimidazole |
|---|---|
| Synonyms |
Bioshik
1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitro-1H-imidazole Trimonase Fasigyn Fasygin TINDAMAX UNII-033KF7V46H 1-(2-(Ethylsulfonyl)ethyl)-2-methyl-5-nitro-1H-imidazole MFCD00057217 Tinidazolum Simplotan Tinidazol Tinidazole Fasigin Tricolam 1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole Ethyl[2-(2-methyl-5-nitro-1-imidazoIyl)ethyl]sulfone Symplotan EINECS 243-014-4 |
| Description | Tinidazole is a synthesized imidazole derivative used in antiprotozoal treatment with antiamebic and antibacterial properties.Target: AntibacterialTinidazole is a 5-nitroimidazole active in vitro against a wide variety of anaerobic bacteria and protozoa. Tinidazole is an effective treatment against anaerobic microorganisms based on its pharmacokinetic characteristics (C(max) 51 microg/ml, t(1/2) 12.5 h) and its excellent in vitro activity. Its long half-life allows once a day regimens. Tinidazole is as effective as metronidazole in the treatment of infections caused by T. vaginalis, giardiasis and amebiasis and bacterial vaginosis, malaria, odontogenic infections, anaerobic bacterial infections (pelvic inflammatory disease, diabetic foot), surgical prophylaxis (abdominal and hysterectomy) and Helicobacter pylori eradication. Tinidazole has recently been resurrected and FDA approved for trichomoniasis and BV in the USA and is being restudied as an alternative to metronidazole for BV. In vitro antimicrobial activity and pharmacokinetics studies indicate that when compared directly with metronidazole, tinidazole has minor but possibly relevant antimicrobial as well as pharmacokinetic advantages. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 528.4±30.0 °C at 760 mmHg |
| Melting Point | 117-121 °C(lit.) |
| Molecular Formula | C8H13N3O4S |
| Molecular Weight | 247.271 |
| Flash Point | 273.4±24.6 °C |
| Exact Mass | 247.062683 |
| PSA | 106.16000 |
| LogP | -0.27 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | Refrigerator |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H312 + H332-H341-H351 |
| Precautionary Statements | P261-P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R40 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NI6255000 |
| HS Code | 2933290090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |