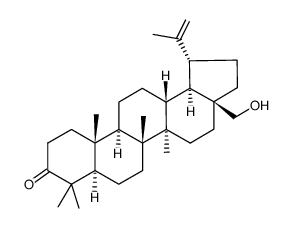
13159-28-9
| Name | Betulinicaldehyde |
|---|---|
| Synonyms |
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carbaldehyde
(1R,3aS,4S,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Hydroxy-1-isopropenyl-5a,5b,8,8,11a-pentamethyl-eicosahydro-cyclopenta[a]chrysene-3a-carbaldehyde (3β)-3-Hydroxylup-20(29)-en-28-al Betulinaldehyde |
| Description | Betulinaldehyde(Betunal) belongs to pentacyclic triterpenoids and was reported to exhibit antimicrobial activities against bacteria and fungi, including S. aureus.IC50 value:Target: Betulinaldehyde(Betunal) belongs to pentacyclic triterpenoids that are based on a 30-carbon skeleton comprising four six-membered rings and one five-membered ring. Betulinaldehyde regulates multiple desirable targets which could be further explored in the development of therapeutic agents for the treatment of S. aureus infections [1]. Study compounds α-amyrin [3β-hydroxy-urs-12-en-3-ol (AM)], betulinic acid [3β-hydroxy-20(29)-lupaene-28-oic acid (BA)] and betulinaldehyde [3β-hydroxy-20(29)-lupen-28-al (BE)] belong to pentacyclic triterpenoids and were reported to exhibit antimicrobial activities against bacteria and fungi, including S. aureus. The MIC values of these compounds against a reference strain of methicillin-resistant S. aureus (MRSA) (ATCC 43300) ranged from 64 μg/ml to 512 μg/ml. However, the response mechanisms of S. aureus to these compounds are still poorly understood [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 513.9±33.0 °C at 760 mmHg |
| Molecular Formula | C30H48O2 |
| Molecular Weight | 440.701 |
| Flash Point | 217.4±18.0 °C |
| Exact Mass | 440.365417 |
| PSA | 37.30000 |
| LogP | 9.07 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Storage condition | -20°C |
|
~17% 
13159-28-9 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 10, # 10 p. 3229 - 3236 |
|
~91% 
13159-28-9 |
| Literature: Csuk, Rene; Schmuck, Kianga; Schaefer, Renate Tetrahedron Letters, 2006 , vol. 47, # 49 p. 8769 - 8770 |
|
~% 
13159-28-9 |
| Literature: STORA ENSO OYJ; WICKHOLM, Niko; ALAKURTTI, Sami; YLI-KAUHALUOMA, Jari; KOSKIMIES, Salme Patent: WO2013/38316 A1, 2013 ; Location in patent: Page/Page column 15 ; |
|
~4% 
13159-28-9 |
| Literature: Phytochemistry (Elsevier), , vol. 36, # 6 p. 1369 - 1380 |
|
~% 
13159-28-9 |
| Literature: WO2008/57420 A2, ; Page/Page column 280-281 ; |
|
~% 
13159-28-9 |
| Literature: Barthel, Alexander; Stark, Sebastian; Csuk, Rene Tetrahedron, 2008 , vol. 64, # 39 p. 9225 - 9229 |
|
~7% 
13159-28-9
Detail
|
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 8, # 13 p. 1707 - 1712 |
|
~35% 
13159-28-9 |
| Literature: Chemistry of Natural Compounds, , vol. 38, # 1 p. 58 - 61 |
|
~% 
13159-28-9 |
| Literature: Helvetica Chimica Acta, , vol. 24, p. 529 Helvetica Chimica Acta, , vol. 25, p. 171 Chemische Berichte, , vol. 65, p. 1305 |