473-98-3
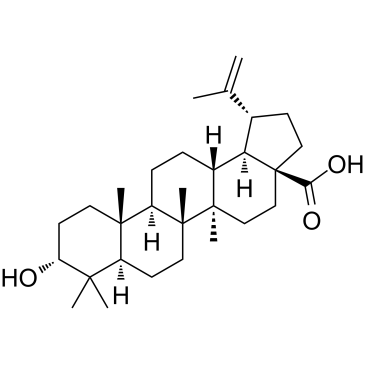
| Name | betulin |
|---|---|
| Synonyms |
Betuline
Lup-20(30)-ene-3b,28-diol TROCHOL 3b-Lup-20(29)-ene-3,28-diol Betulin BETULOL (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a-(Hydroxyméthyl)-5a,5b,8,8,11a-pentaméthyl-1-(1-propèn-2-yl)icosahydro-1H-cyclopenta[a]chrysén-9-ol (3β)-Lup-20(29)-ene-3,28-diol MFCD00016802 Lup-20(29)-ene-3b,28-diol betula camphor BETULINOL (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a-(Hydroxymethyl)-1-isopropenyl-5a,5b,8,8,11a-pentamethylicosahydro-1H-cyclopenta[a]chrysen-9-ol EINECS 207-475-5 Lup-20(30)-ene-3β,28-diol |
| Description | Betulin (Trochol), is a sterol regulatory element-binding protein (SREBP) inhibitor with an IC50 of 14.5 μM in K562 cell line. |
|---|---|
| Related Catalog | |
| Target |
IC50: 14.5 μM (SREBP, K562 cell), 74.1 μM (SREBP, HeLa cell), 17.1 μM (SREBP, GOTO cell)[1], 21.09 μM (SREBP, 181P cell), 20.62 μM (SREBP, HeLa cell)[2] |
| In Vitro | Betulin (BE) displays a broad spectrum of biological and pharmacological properties, among which the anticancer and chemopreventive activity attract most of the attention. BE has been shown to elicit anticancer properties by inhibiting cancer cells growth. BE has exhibited quite a different range of its antiproliferative activity, depending on cancer cells type, from a weak inhibition of cell proliferation in human erythroleukaemia cell line (K562) to a strong inhibition in human neuroblastoma cells (SK-N-AS), where the effect has been most pronounced. Additionally, BE has also been found to express significant cytotoxicity against primary cancer cells cultures isolated from tumour samples obtained from ovarian, cervical carcinoma, and glioblastoma patients, where the IC50 values have ranged from 2.8 to 3.4 μM, being significantly lower, when compared with established cell lines[1]. The cytotoxic activity of crude birch bark extract and purified betulin and betulinic acid towards human gastric carcinoma (EPG85-257) and human pancreatic carcinoma (EPP85-181) drug-sensitive and drug-resistant (daunorubicin and mitoxantrone) cell lines are compared. Significant differences in sensitivity between cell lines depending on the compound used are shown, suggesting that both betulin and betulinic acid can be considered as a promising leads in the treatment of cancer[2]. |
| In Vivo | Betulin could improve glucose intolerance and modify basal learning performance. Treatment with betulin significantly restores SOD activity and decreased MDA content in hippocampus. Betulin also markedly reduces the contents of inflammatory cytokines in serum and hippocampus. Furthermore, administration of BE effectively upregulated the expressions of Nrf2, HO-1 and blocked the phosphorylations of IκB, NF-κB. In summary, BE might exhibit protective effect on cognitive decline in STZ-induced diabetic rats through HO-1/Nrf-2/ NF-κB pathway[3]. |
| Cell Assay | Chemoresistance is tested using a proliferation assay based on sulphorhodamine B staining. Briefly, 800 cells per well are seeded in triplicate in 96-well plates. After attachment for 24 h, substances are added in dilution series for a 5-day incubation, before SRB staining is performed. Incubation is terminated by replacing the medium with 10% trichloroacetic acid, followed by further incubation at 4°C for 1h. Subsequently, the plates are ished five times with water and stained by adding 100 μL 0.4% SRB in 1% acetic acid for 10 min at room temperature. Ishing the plates five times with 1% acetic acid eliminated unbound dye. After air-drying and re-solubization of the protein bound dye in 10 mM Tris-HCl (pH=8.0), absorbance is read at 562 nm[2]. |
| Animal Admin | Rats: The rats are randomLy divided into five groups (n=10): control group, STZ group, STZ+betulin (20 mg/kg) group, STZ+betulin (40 mg/kg) group. Diabetes is induced by STZ (30 mg/kg, i.p.) dissolved in citrate buffer (pH 4.4, 0.1 M) using 1 mL syringe for 4 weeks, meanwhile the control rats receive an equal volume of citrate buffer. Thereafter, the diabetic rats are treated with betulin (20 mg/kg, 40 mg/kg) for another 4 weeks[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 522.3±23.0 °C at 760 mmHg |
| Melting Point | 256-257 °C(lit.) |
| Molecular Formula | C30H50O2 |
| Molecular Weight | 442.717 |
| Flash Point | 210.9±17.2 °C |
| Exact Mass | 442.381073 |
| PSA | 40.46000 |
| LogP | 9.01 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.525 |
| Storage condition | 2-8°C |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H371 |
| Precautionary Statements | P260 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36/37-S36-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | OK5755000 |
| HS Code | 29181985 |
| Precursor 7 | |
|---|---|
| DownStream 9 | |
| HS Code | 29181985 |
|---|