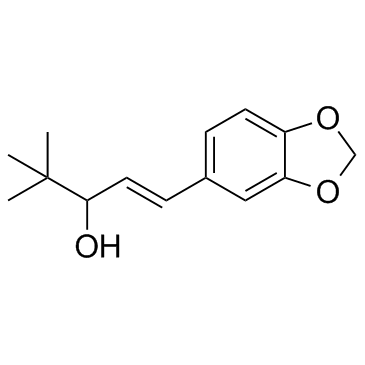
49763-96-4
| Name | Stiripentol |
|---|---|
| Synonyms |
1-(1,3-Benzodioxol-5-yl)-4,4-dimethyl-1-penten-3-ol
4,4-Dimethyl-1-(3,4-methylenedioxyphenyl)-1-penten-3-ol 4,4-dimethyl-1-[3,4(methylenedioxy)-phenyl]-1-penten-3-ol,Diacomit Stiripentol Stiripentol [USAN:INN] (1E)-1-(1,3-Benzodioxol-5-yl)-4,4-dimethyl-1-penten-3-ol (E)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol UNII:R02XOT8V8I |
| Description | Stiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to NCLB mediatated by CYP3A4 (noncompetitively) and CYP2C19 (competitively) with Ki of 1.59±0.07 and 0.516±0.065 μM and IC50 of 1.58 and 3.29 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.58 μM (CYP3A4), 3.29 μM (CYP2C19)[1] Ki: 1.59±0.07μM (CYP3A4), 0.516±0.065 μM (CYP2C19)[1] |
| In Vitro | Stiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to N-desmethylclobazam (NCLB) mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively). The inhibition of CLB demethylation by Stiripentol (STP) is best described by a noncompetitive inhibition model with apparent Ki=1.6 μM for the cDNA-expressing CYP3A4 and by a competitive inhibition model with Ki=0.52 μM for the cDNA-expressing CYP2C19. Formation of OH-NCLB from NCLB by cDNA-expressing CYP2C19 is competitively inhibited by Stiripentol (STP) with a Ki=0.14 μM[1]. |
| In Vivo | In mice treating with Stiripentol (STP) monotherapy, the difference between BT1 (39.67±1.09°C) and BT2 (41.32±1.05°C) reaches statistical significance (t=3.097, p<0.05). The difference in BT2 between Stiripentol (STP) monotherapy and CLB monotherapy is statistically significant (t=2.615, p<0.05). In mice treating with Stiripentol (STP)+CLB combination therapy, the difference between BT1 (40.18±0.58°C) and BT2 (43.03±0.49°C) reaches statistical significance (t=10.44, p<0.01)[2]. |
| Cell Assay | The inhibition constants (apparent Ki) of Stiripentol (STP) for CLB demethylation by CYP3A4 and CYP2C19 are determined using various concentrations of CLB (2, 10, 20, 40, 60, and 100 μM) with increasing concentrations of Stiripentol (STP) (0, 0.5, 1, 2, and 5 μM). Concerning NCLB hydroxylation by CYP2C19, the apparent Ki is similarly determined with different concentrations of NCLB (1.5, 4, 6, 8, 12, and 14 μM) and STP (0, 0.1, 0.5, 1, and 2 μM). IC50 values are determined by coincubation of the substrate at concentration in the range of the therapeutic plasma concentrations (2 μM CLB or 14 μM NCLB) with increasing concentrations of Stiripentol (STP) (0.001, 0.002, 0.005, 0.01, 0.05, 0.1, 0.25, 2, 5, and 10 μM)[1]. |
| Animal Admin | Two age groups, p1M (n=18, age 4 weeks) and p5M (n=18, age 5-10 months), of Scn1aRX/+ mice are assigned in this experiment. Both groups are divided randomly into three subgroups (n=6), and each subgroup is administered Stiripentol (STP) (300 mg/kg) alone, CLB (6.62 mg/kg) alone, or a combination of Stiripentol (STP) (p1M; 150 mg/kg, p5M; 300 mg/kg) and CLB (6.62 mg/kg). All drugs are administered by intraperitoneal injection (i.p.) after a 48-h recovery from baseline seizure study. Blood samples are collected at 1 h and 20 min after administration of CLB or STP+CLB for measurement of plasma concentrations of CLB and N-desmethylclobazam, respectively[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 365.4±11.0 °C at 760 mmHg |
| Melting Point | 73-74ºC |
| Molecular Formula | C14H18O3 |
| Molecular Weight | 234.291 |
| Flash Point | 174.8±19.3 °C |
| Exact Mass | 234.125595 |
| PSA | 38.69000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.579 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|