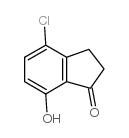
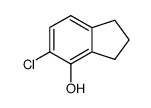
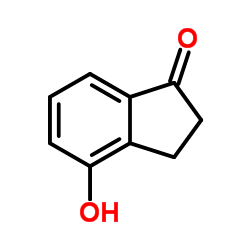
145-94-8
| Name | 7-chloro-2,3-dihydro-1H-inden-4-ol |
|---|---|
| Synonyms |
7-Chloro-4-indanol
7-Chlor-indanol-(4) 4-Indanol,7-chloro CHLORINDANOL Clorindanolum [INN-Latin] 7-Chlor-indan-4-ol Lanesta 7-chloro-indan-4-ol Clorindanol |
| Description | Chlorindanol is a new antiseptic agent. |
|---|---|
| Related Catalog |
| Density | 1.308g/cm3 |
|---|---|
| Boiling Point | 290ºC at 760mmHg |
| Melting Point | 91-93ºC |
| Molecular Formula | C9H9ClO |
| Molecular Weight | 168.62000 |
| Flash Point | 129.2ºC |
| Exact Mass | 168.03400 |
| PSA | 20.23000 |
| LogP | 2.53430 |
| Vapour Pressure | 0.00122mmHg at 25°C |
| Index of Refraction | 1.618 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|
|
~% 
145-94-8 |
| Literature: US2012/252758 A1, ; Page/Page column 69 ; |
|
~% 
145-94-8 |
| Literature: Journal of the American Chemical Society, , vol. 79, p. 3559 DE951629 , ; |
|
~% 
145-94-8 |
| Literature: Journal of the American Chemical Society, , vol. 79, p. 3559 |
|
~% 
145-94-8 |
| Literature: US2012/252758 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |