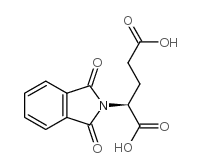
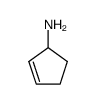
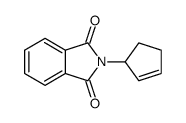
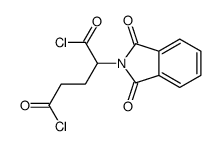
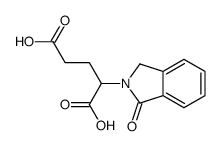
6349-98-0
| Name | 2-Phthalimidoglutaric acid |
|---|---|
| Synonyms |
N-Phthalimido-glutamicacid
PHT-DL-GLU-OH Einecs 218-952-2 Glutamic acid,phthalyl N,N-phthaloyl-DL-glutamic acid N-phthalylglutamic acid 2-phthalimido-glutaric acid Phthalimidoglutaric acid Ba 2733 Einecs 228-754-8 Phthalylglutamic acid PHTHALOYL-DL-GLUTAMIC ACID |
| Description | 2-(1,3-Dioxoisoindolin-2-yl)pentanedioic acid is a glutamic acid derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | 192 °C |
|---|---|
| Molecular Formula | C13H11NO6 |
| Molecular Weight | 277.23000 |
| Exact Mass | 277.05900 |
| PSA | 111.98000 |
| LogP | 0.53850 |
| RTECS | MA3810000 |
|---|
|
~% 
6349-98-0 |
| Literature: Billman; Harting Journal of the American Chemical Society, 1948 , vol. 70, p. 1473 Full Text View citing articles Show Details King; Kidd Journal of the Chemical Society, 1949 , p. 3315,3318 |
|
~% 
6349-98-0 |
| Literature: Schilling; Strong Journal of the American Chemical Society, 1955 , vol. 77, p. 2843 |
|
~% 
6349-98-0 |
| Literature: King; Kidd Journal of the Chemical Society, 1949 , p. 3315,3318 |
|
~% 
6349-98-0 |
| Literature: Crossley, Maxwell J.; Fung, Yik M.; Kyriakopoulos, Efstathia; Potter, Jeffrey J. Journal of the Chemical Society - Perkin Transactions 1, 1998 , # 6 p. 1123 - 1130 |
|
~% 
6349-98-0 |
| Literature: Du Pont de Nemours and Co. Patent: US2801250 , 1955 ; |
|
~% 
6349-98-0 |
| Literature: Du Pont de Nemours and Co. Patent: US2801250 , 1955 ; |
|
~% 
6349-98-0 |
| Literature: Du Pont de Nemours and Co. Patent: US2801250 , 1955 ; |
|
~% 
6349-98-0 |
| Literature: Du Pont de Nemours and Co. Patent: US2801250 , 1955 ; |
| Precursor 6 | |
|---|---|
| DownStream 6 | |