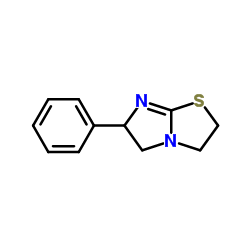
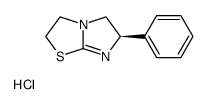
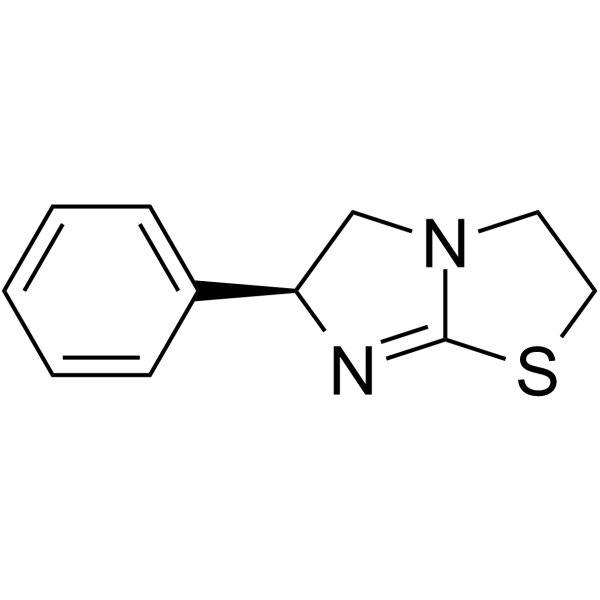
16595-80-5
| Name | Levamisole hydrochloride |
|---|---|
| Synonyms |
(-)-Tetramisole hydrochloride
Solaskil Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl-, (6S)-, hydrochloride (1:1) Spartakon Levamisol hydrochloride (-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole hydrochloride (6S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazolhydrochlorid (S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole hydrochloride Ascaridil Levasole Meglum Levadin MFCD00005536 (S)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole Monohydrochloride L[-]-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole (-)-2,3,5,6-Tetrahydro-6-phenylimidazo(2,1-b)thiazole monohydrochloride Decaris L-(-)-2,3,5,6-Tetrahydro-6-phenyl-imidazo(2,1-b)thiazole hydrochloride (-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole monohydrochloride Nilverm Ripercol L(−)-2,3,5,6-Tetrahydro-6-phenylimidazo(2,1-b)thiazole hydrochloride Levamisole HCl L-(-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole Monohydrochloride EINECS 240-654-6 Tramisol (6S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole hydrochloride (1:1) L-Tetramisole hydrochloride Levamisole hydrochloride Levacide Ergamisol Nemicide (6S)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole hydrochloride Levamisole (hydrochloride) |
| Description | Levamisole Hcl is an anthelmintic and immunomodulator belonging to a class of synthetic imidazothiazole derivatives.IC50 value: Target: Levamisole suppresses the production of white blood cells, resulting in neutropenia and agranulocytosis. With the increasing use of levamisole as an adulterant, a number of these complications have been reported among cocaine users [1] [2]. Levamisole reversibly and noncompetitively inhibits most isoforms of alkaline phosphatase (e.g., human liver, bone, kidney, and spleen) except the intestinal and placental isoform [3]. It is thus used as an inhibitor along with substrate to reduce background alkaline phosphatase activity in biomedical assays involving detection signal amplification by intestinal alkaline phosphatase, for example in in situ hybridization or Western blot protocols. It is used to immobilize the nematode C. elegans on glass slides for imaging. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 344.4ºC at 760 mmHg |
|---|---|
| Melting Point | 226-231ºC |
| Molecular Formula | C11H13ClN2S |
| Molecular Weight | 240.752 |
| Flash Point | 162.1ºC |
| Exact Mass | 240.048798 |
| PSA | 40.90000 |
| LogP | 2.32160 |
| Index of Refraction | -126 ° (C=1, H2O) |
| Storage condition | 2~8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S28-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | NJ5960000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933290090 |
|
~% 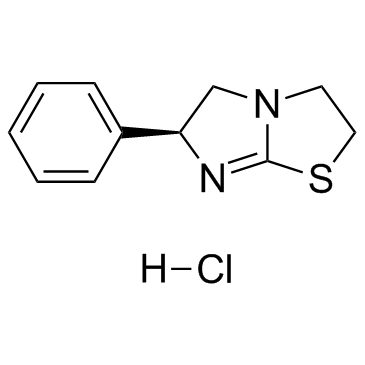
16595-80-5 |
| Literature: Tetrahedron, , vol. 41, # 12 p. 2465 - 2470 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |