180200-66-2
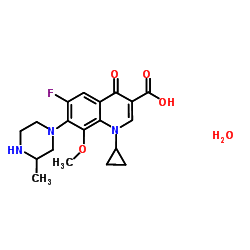
| Name | 1-Cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid sesquihydrate |
|---|---|
| Synonyms |
1-CYCLOPROPYL-6-FLUORO-1,4-DIHYDRO-8-METHOXY-7-(3-METHYLPIPERAZIN-1-YL)-4-OXO-3-QUINOLINECARBOXYLIC ACID
Gatifloxacin formic acid ethyl ester 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-, hydrate (2:3) Gatifloxacin (300 mg) TEQUIN UNII:L4618BD7KJ 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic acid trihydrate Gatifloxacin Tablet Gatifloxacin Monohydrate 1-Cyclopropyl-6-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylic acid Gatifloxacin sesquihydrate 1-Cyclopropyl-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid hydrate (2:3) |
| Description | Gatifloxacin sesquihydrate (AM-1155; BMS-206584; PD135432) is a potent fluoroquinolone antibiotic with broad-spectrum antibacterial activity. Gatifloxacin sesquihydrate inhibits bacterial type II topoisomerases (IC50=13.8 μg/ml for S. aureus topoisomerase IV) and E. coli DNA gyrase (IC50 = 0.109 μg/ml)[1]. Gatifloxacin sesquihydrate can be used to treat bacterial conjunctivitis in vivo. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II:36.7 μM (IC50) |
| In Vitro | Gatifloxacin sesquihydrate is against S. aureus MS5935 topoisomerase IV, E. coli NIHJ JC-2 DNA gyrase and HeLa cell topoisomerase II with IC50 values of 13.8 μg/ml, 0.109 μg/ml, and 265 μg/ml, respectively[1]. Gatifloxacin sesquihydrate is against S. aureus MS5935 topoisomerase IV, E. coli NIHJ JC-2 DNA gyrase and HeLa cell topoisomerase II with MIC values of 0.05 μg/ml, 0.0063 μg/ml, and 122 μg/ml, respectively[1]. Gatifloxacin sesquihydrate exhibits antibacterial activities for wild-type strains (MS5935, MS5952, MR5867 and MR6009) the first-, second-, third-, and fourth-step mutants with MIC values of 0.05 to 0.10 μg/ml, 0.20 μg/ml, 1.56 to 3.13 μg/ml, 1.56 to 6.25 μg/ml, and 50 to 200 μg/ml, respectively. Gatifloxacin sesquihydrate displays the most potent activity against the second- and third-step mutants (MS5952, MR5867 and MR6009) except for the second-step mutant of strain MS5935[2]. Gatifloxacin sesquihydrate has potent activity against norA transformant NY12 (MIC, 0.39 μg/ml)[2]. Gatifloxacin sesquihydrate (20-100 μM; 72 hours) significantly decreases insulin content to 60% at Day 1, and continues to be reduced to 50.1% and 44.7% at Day 3 by 20 μM and 100 μM Gatifloxacin sesquihydrate, respectively[3]. |
| In Vivo | Gatifloxacin sesquihydrate (subcutaneous injection; 100 mg/kg; 3 times a day; 30 days) significantly decreases the number of lesions in mouse footpad with Nocardia brasiliensis[4]. Animal Model: Female BALB/c mice with Nocardia brasiliensis in the right hind footpad[4] Dosage: 100 mg/kg Administration: Subcutaneous injection; 3 times a day; 30 days Result: Reduced the production of lesions in mice. |
| References |
| Density | 1.386 g/cm3 |
|---|---|
| Boiling Point | 607.8ºC at 760 mmHg |
| Molecular Formula | C19H22FN3O4.3/2H2O |
| Molecular Weight | 804.834 |
| Flash Point | 321.4ºC |
| Exact Mass | 804.350586 |
| PSA | 195.29000 |
| LogP | 4.55550 |
| Vapour Pressure | 6.43E-23mmHg at 25°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |