15266-38-3
| Name | (Z)-1-Methyl-2-(tridec-8-en-1-yl)quinolin-4(1H)-one |
|---|---|
| Synonyms |
1-Methyl-2-[(8Z)-8-tridecen-1-yl]-4(1H)-quinolinone
evocarpine |
| Description | Evocarpine, a quinolone alkaloid that could be isolated from Evodiae fructus, inhibitss Ca2+ influx through voltage-dependent calcium channels. Antimycobacterial activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cumulative application of evocarpine (1-100 μM) inhibits the sustained contraction induced by 60 mM K+ in a concentration-dependent manner[1]. Evocarpine is found to induce apoptotic cell death in promyelocytic leukaemia HL‐60 cells in dose- and time-dependent manners[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.2±45.0 °C at 760 mmHg |
| Melting Point | 34-38℃ |
| Molecular Formula | C23H33NO |
| Molecular Weight | 339.514 |
| Flash Point | 156.1±18.1 °C |
| Exact Mass | 339.256226 |
| PSA | 22.00000 |
| LogP | 7.70 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.526 |
| Storage condition | 2-8C |
|
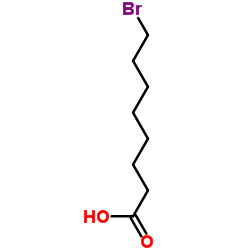
~90% 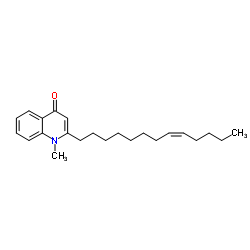
15266-38-3 |
| Literature: Coppola, Gary M. Journal of Heterocyclic Chemistry, 1985 , vol. 22, p. 491 - 494 |
|
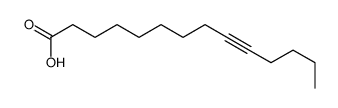
~% 
15266-38-3 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 22, p. 491 - 494 |
|
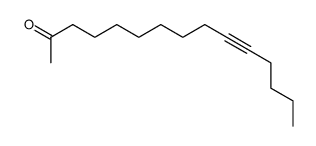
~% 
15266-38-3 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 22, p. 491 - 494 |
|
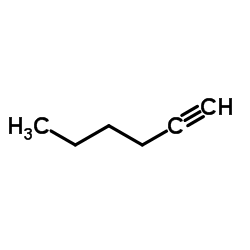
~% 
15266-38-3 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 22, p. 491 - 494 |
|
~% 
15266-38-3 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 22, p. 491 - 494 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |