CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YQ1170000
-
CHEMICAL NAME :
-
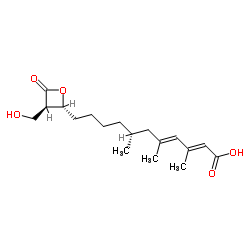
2,4-Undecadienoic acid, 11-(3-(hydroxymethyl)-4-oxo-2-oxetanyl)-3,5,7-trimeth yl-, (2R-(2-alpha(2E,4E,7R*),3-beta))-
-
CAS REGISTRY NUMBER :
-
29066-42-0
-
LAST UPDATED :
-
199406
-
DATA ITEMS CITED :
-
2
-
MOLECULAR FORMULA :
-
C18-H28-O5
-
MOLECULAR WEIGHT :
-
324.46
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JANTAJ Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo, 141, Japan) V.2-5, 1948-52; V.21- 1968- Volume(issue)/page/year: 41,247,1988
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JANTAJ Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo, 141, Japan) V.2-5, 1948-52; V.21- 1968- Volume(issue)/page/year: 41,247,1988
|
![Diphenylmethyl [2E,4E,7R,(2'R,3'R)]-11-(3'-hydroxymethyl-4'-oxooxetan-2'-yl)-3,5,7-trimethylundeca-2,4-dienoate structure](https://image.chemsrc.com/caspic/343/247166-82-1.png)
