hymeglusin
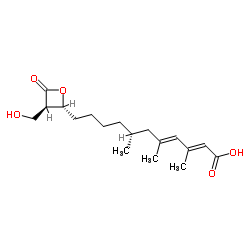
hymeglusin structure
|
Common Name | hymeglusin | ||
|---|---|---|---|---|
| CAS Number | 29066-42-0 | Molecular Weight | 324.412 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 516.6±15.0 °C at 760 mmHg | |
| Molecular Formula | C18H28O5 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 181.3±13.9 °C | |
Use of hymeglusinHymeglusin, as a fungal β-lactone antibiotic, is a HMG-CoA synthase inhibitor (IC50 = 0.12 μM). Hymeglusin covalently modifies the active Cys129 residue of the enzyme[2][3]. |
| Name | Hymeglusin |
|---|---|
| Synonym | More Synonyms |
| Description | Hymeglusin, as a fungal β-lactone antibiotic, is a HMG-CoA synthase inhibitor (IC50 = 0.12 μM). Hymeglusin covalently modifies the active Cys129 residue of the enzyme[2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.2 μM (HMG-CoA)[2][3] |
| In Vitro | Hymeglusin inhibits dengue type 2 (DEN-2) New Guinea C (NGC) live virus replication in K562 cells. The therapeutic index (CC50/IC50) of Hymeglusin is 11. The EC50 of Hymeglusin in K562 cells is 4.5 μM[1]. |
| In Vivo | At a dose of 25 mg/kg, hymeglusin inhibits cholesterol biosynthesis in rats by 45 %[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 516.6±15.0 °C at 760 mmHg |
| Molecular Formula | C18H28O5 |
| Molecular Weight | 324.412 |
| Flash Point | 181.3±13.9 °C |
| Exact Mass | 324.193665 |
| PSA | 83.83000 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.510 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | YQ1170000 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Characterization of peroxisomal 3-hydroxy-3-methylglutaryl coenzyme A reductase in UT2 cells: sterol biosynthesis, phosphorylation, degradation, and statin inhibition.
Biochemistry 39(1) , 237-47, (2000) We have previously identified a CHO cell line (UT2 cells) that expresses only one 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase protein which is localized exclusively in peroxisomes [Engfe... |
|
|
Binding site for fungal beta-lactone hymeglusin on cytosolic 3-hydroxy-3-methylglutaryl coenzyme A synthase.
Biochim. Biophys. Acta 1636(1) , 22-8, (2004) We studied the molecular mechanism through which the fungal beta-lactone, hymeglusin, potently and specifically inhibits 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase. [(14)C]Hymeglusin covalently... |
|
|
Biosynthesis of antibiotic 1233A (F-244) and preparation of [14C]1233A.
J. Antibiot. 45(4) , 563-7, (1992) The biosynthesis of antibiotic 1233A (F-244) was studied by feeding 13C-labeled precursors to the producing organism, Scopulariopsis sp. F-244. 13C NMR spectroscopy established that 1233A is derived f... |
| (2E,4E,7R)-11-[(2R,3R)-3-(Hydroxymethyl)-4-oxo-2-oxetanyl]-3,5,7-trimethyl-2,4-undecadienoic acid |
| 2,4-Undecadienoic acid, 11-[(2R,3R)-3-(hydroxymethyl)-4-oxo-2-oxetanyl]-3,5,7-trimethyl-, (2E,4E,7R)- |
| hymeglusin |
![Diphenylmethyl [2E,4E,7R,(2'R,3'R)]-11-(3'-hydroxymethyl-4'-oxooxetan-2'-yl)-3,5,7-trimethylundeca-2,4-dienoate Structure](https://image.chemsrc.com/caspic/343/247166-82-1.png) CAS#:247166-82-1
CAS#:247166-82-1 CAS#:2385-77-5
CAS#:2385-77-5