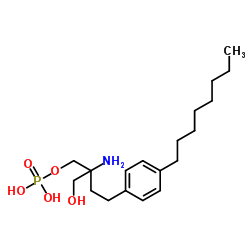
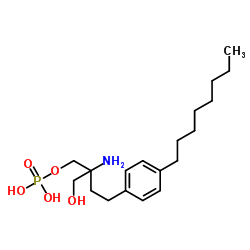
162359-55-9
| Name | fingolimod |
|---|---|
| Synonyms |
2-Amino-2-(4-octylphenethyl)propane-1,3-diol
2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol Fingolimod 2-Amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol fingolimod; Gilenya UNII-3QN8BYN5QF Fingolimod Base |
| Description | Fingolimod is a sphingosine 1-phosphate (S1P) antagonist with IC50 of 0.033 nM in K562 and NK cells. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.033 nM (S1P, in K562 and NK cells)[1] |
| In Vitro | The monocyte-derived immature dendritic cells (iDCs) are pretreated with various concentrations of S1P for various periods of time prior to their incubation with NK cells. Four hours incubation of autologous or allogeneic iDCs with 0.2-20 μM of S1P significantly protectes these cells from NK cell lysis. The IC50 values of S1P are calculated at 160 nM for autologous iDCs, and 34 nM for allogeneic iDCs. Next, the inhibitory effect of S1P is revered by various concentrations of Fingolimod or SEW2871, with an IC50 effect of 173 or 15 nM, respectively[1]. Fingolimod has been reported to reduce LPA synthesis via inhibition of the lysophospholipase autotaxin. Fingolimod treatment correlates with a significant elevation of axonal cAMP, a crucial factor for axonal outgrowth. Additionally, Fingolimod significantly reduces LPA levels in the injured nerve. PF-8380 treatment correlates with improved myelin thickness[2]. |
| In Vivo | Fingolimod treatment results in significantly increased nerve conduction at 14 days post-crush in wildtype C57BL/6 mice. However, Foxn1-/- mice, which are devoid of T- but not B-lymphocytes, show an improvement of nerve regeneration under fingolimod treatment. Although the mean increase in nerve conduction velocity in both fingolimod-treated and controlFoxn1-/- mice implies a potentially positive role of T-lymphocyte deficiency on nerve regeneration, only fingolimod-treated Foxn1-/- mice show a significant improvement compared to C57BL/6 controls and performed better in the functional analysis[2]. Treatment of the animals with Fingolimod for 28 d results in a clear reduction in the binding of 18F-GE180 when compare with vehicle-treated animals and evaluated by ex vivo autoradiography. Quantification of the binding of the radiotracer revealed a significant reduction in the binding potential of 18F-GE180 (P<0.0001) after treatment with Fingolimod[3]. |
| Cell Assay | Immature dendritic cells (DCs) are left intact or are incubated with 2 μM S1P, 10 nM Fingolimod, 10 nM SEW2871 or the combinations of S1P with these drugs for 4 h. As a control 1 μg/mL LPS is used. The cells are washed and incubated in a 96-well plate (v-bottom, 2×105 cells per well), washed again and resuspended in PBS buffer containing 0.1% sodium azide. They are labeled with 1 μg/mL FITC-conjugated mouse anti-human CD80, 1 μg/mL FITC-conjugated mouse anti-human CD83, 1 μg/mL FITC-conjugated mouse anti-human CD86, 1 μg/mL FITC-conjugated mouse anti-human HLA-class I, 1 μg/mL FITC-conjugated mouse anti-human HLA-DR, 1 μg/mL FITC-conjugated mouse anti-human HLA-E, or 1 μg/mL FITC-conjugated mouse IgG as a control. The cells are washed twice, and examined in the flow cytometer. Markers are set according to the isotype control FITC-conjugated mouse IgG[1]. |
| Animal Admin | Mice[2] Rag1-/- mice(B6.129S7-Rag1tm1Mom/J), Foxn1-/- mice (B6.Cg/NTac-Foxn1nu) mice, and control C57BL/6 mice are used. Mice receive non-phosphorylated Fingolimod via intraperitoneal injection at a concentration of 1 mg/kg once daily over the course of 16 days, starting 2 days before crush until 14 days post-crush. PF-8380 is dissolved in DMSO and administered at a concentration of 10 mg/kg via intraperitoneal injection once daily, as well starting 2 days before until 14 days post-crush. Controls receive an equal volume of solvent. Rats[3] Male Lewis rats (50-100 g, n=18) are used. After the peripheral activation of the lesion, the DTH EAE lesions are allowed to develop until day 127 to generate large chronic lesions and the animals are then treated for 28 d with either Fingolimod (n=7) or vehicle (n=7). The Fingolimod treatment is given daily (0.3 mg/kg in 0.5 mL of water). The control group is given water (0.5 mL) as a vehicle control. The animals are dosed via oral gavage to ensure accurate dosing. In this model, the acute inflammation subsides after day 20 of activation of the lesion, and the BBB damage subsides. Thus, at day 127 the lesion clearly represents a well-developed chronic MS lesion. PET imaging is performed immediately before initiation of treatment and at the end of the treatment period. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 479.5±45.0 °C at 760 mmHg |
| Molecular Formula | C19H33NO2 |
| Molecular Weight | 307.471 |
| Flash Point | 243.8±28.7 °C |
| Exact Mass | 307.251129 |
| PSA | 66.48000 |
| LogP | 5.25 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.531 |
| Storage condition | 2-8°C |
| Precursor 7 | |
|---|---|
| DownStream 5 | |
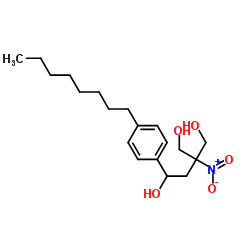
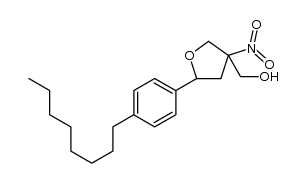
![N-[2,2-Dimethyl-5-[2-(4-octylphenyl)ethyl]-1,3-dioxan-5-yl]carbamic acid 1,1-dimethylethyl ester structure](https://image.chemsrc.com/caspic/224/885605-36-7.png)

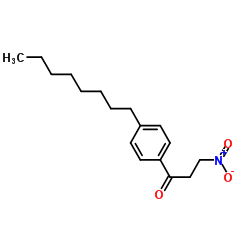
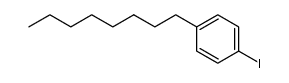
![[2-methyl-4-[2-(4-octylphenyl)ethyl]-5H-1,3-oxazol-4-yl]methanol structure](https://image.chemsrc.com/caspic/165/402616-28-8.png)
![[1,1-Bis(hydroxymethyl)-3-(4-octylphenyl)propyl]carbamic acid Phenylmethyl Ester structure](https://image.chemsrc.com/caspic/017/402616-41-5.png)