402615-91-2
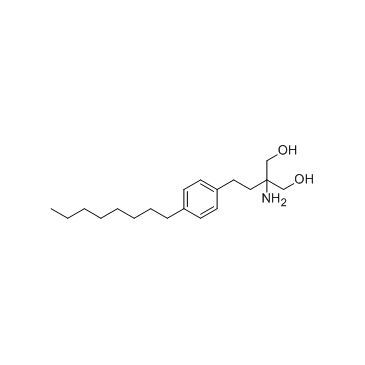
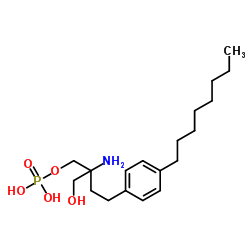
| Name | 2-Amino-2-(hydroxymethyl)-4-(4-octylphenyl)butyl dihydrogen phosp hate |
|---|---|
| Synonyms |
1,3,4,5,6-pentahydroxyl-2-hexanone
rac FTY720 Phosphate phosphoric acid mono-[(R/S)-2-amino-2-hydroxymethyl-4-(4-octyl-phenyl)-butyl] ester Frutabs fingolimod-P 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol mono(dihydrogenphosphate) ester 1,3-Propanediol, 2-amino-2-[2-(4-octylphenyl)ethyl]-, mono(dihydrogen phosphate) (ester) fingolimod phosphate levugen FTY720-phosphate laevosan keto D-fructose Fruit sugar laevoral 2-Amino-2-(hydroxymethyl)-4-(4-octylphenyl)butyl dihydrogen phosphate Fructosteril 2-ammonio-2-(hydroxymethyl)-4-(4-octylphenyl)butyl hydrogen phosphate (D)-fructose fructose powder |
| Description | Fingolimod phosphate (FTY720 phosphate) is an orally active sphingosine 1-phosphate (S1P) receptor agonist. Fingolimod phosphate can promote the neuroprotective effects of microglia. Fingolimod phosphate can be used for the research of multiple sclerosis and neurologic diseases[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Fingolimod phosphate 可防止淋巴细胞移出淋巴器官并抑制自身反应性淋巴细胞浸润中枢神经系统[1]。 Fingolimod phosphate(0、1、10、100 nM)结合 S1P1 受体,下调激活的小胶质细胞产生促炎细胞因子,如肿瘤坏死因子-a、白介素-1β 和白介素-6[1]. Fingolimod phosphate(0、1、10、100 nM)也上调小胶质细胞产生脑源性神经营养因子和胶质细胞源性神经营养因子[1]。 |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 584.2±60.0 °C at 760 mmHg |
| Melting Point | 122-124ºC |
| Molecular Formula | C19H34NO5P |
| Molecular Weight | 387.451 |
| Flash Point | 307.1±32.9 °C |
| Exact Mass | 387.217468 |
| PSA | 122.82000 |
| LogP | 4.27 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.541 |
| Storage condition | 2-8℃ |
|
~% 
402615-91-2 |
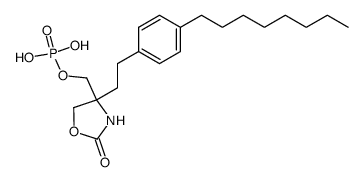
| Literature: Albert, Rainer; Hinterding, Klaus; Brinkmann, Volker; Guerini, Danilo; Mueller-Hartwieg, Constanze; Knecht, Helmut; Simeon, Corinne; Streiff, Markus; Wagner, Trixie; Weizenbach, Karl; Zecri, Frederic; Zollinger, Markus; Cooke, Nigel; Francotte, Eric Journal of Medicinal Chemistry, 2005 , vol. 48, # 16 p. 5373 - 5377 |
|
~% 
402615-91-2 |
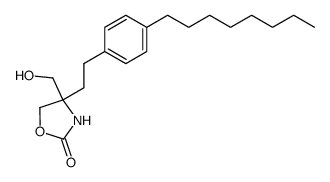
| Literature: Albert, Rainer; Hinterding, Klaus; Brinkmann, Volker; Guerini, Danilo; Mueller-Hartwieg, Constanze; Knecht, Helmut; Simeon, Corinne; Streiff, Markus; Wagner, Trixie; Weizenbach, Karl; Zecri, Frederic; Zollinger, Markus; Cooke, Nigel; Francotte, Eric Journal of Medicinal Chemistry, 2005 , vol. 48, # 16 p. 5373 - 5377 |
|
~% 
402615-91-2 |
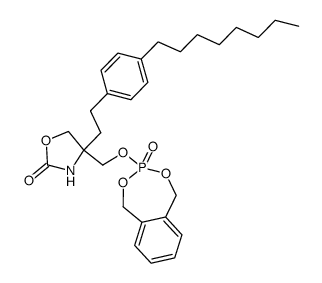
| Literature: Albert, Rainer; Hinterding, Klaus; Brinkmann, Volker; Guerini, Danilo; Mueller-Hartwieg, Constanze; Knecht, Helmut; Simeon, Corinne; Streiff, Markus; Wagner, Trixie; Weizenbach, Karl; Zecri, Frederic; Zollinger, Markus; Cooke, Nigel; Francotte, Eric Journal of Medicinal Chemistry, 2005 , vol. 48, # 16 p. 5373 - 5377 |
|
~% 
402615-91-2 |
| Literature: Albert, Rainer; Hinterding, Klaus; Brinkmann, Volker; Guerini, Danilo; Mueller-Hartwieg, Constanze; Knecht, Helmut; Simeon, Corinne; Streiff, Markus; Wagner, Trixie; Weizenbach, Karl; Zecri, Frederic; Zollinger, Markus; Cooke, Nigel; Francotte, Eric Journal of Medicinal Chemistry, 2005 , vol. 48, # 16 p. 5373 - 5377 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |