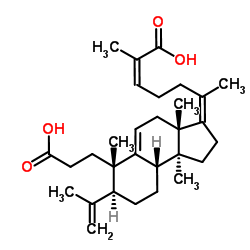
1016260-22-2
| Name | (2Z,6Z)-6-[(3aS,6S,7S,9bS)-6-(2-Carboxyethyl)-7-isopropenyl-3a,6, 9b-trimethyl-1,2,3a,4,6,7,8,9,9a,9b-decahydro-3H-cyclopenta[a]nap hthalen-3-ylidene]-2-methyl-2-heptenoic acid |
|---|---|
| Synonyms |
(2Z,6Z)-6-[(3aS,6S,7S,9aS,9bS)-6-(2-Carboxyethyl)-7-isopropenyl-3a,6,9b-trimethyl-1,2,3a,4,6,7,8,9,9a,9b-decahydro-3H-cyclopenta[a]naphthalen-3-ylidene]-2-methyl-2-heptenoic acid
5'-CMP 5'-CYTIDYLIC ACID CMP MONOHYDRATE 2',3'-dideoxy-[5']cytidylic acid 3,4-seco-lanosta-4(28),9(11),17(20),17(Z),24(Z)-tetraene-3,26-dioic acid Kadsuracoccinic acid A K-2',3'-dideoxy-cytidine monophosphate C-5-P 5'-CYTIDYLIC ACID MONOHYDRATE 5'-CYTIDINE MONOPHOSPHATE |
| Description | Kadsuracoccinic acid A is a tetracyclic natural compound that can be isolated from the stems of Kadsura coccinea. Kadsuracoccinic acid A has vitro anti-HIV-1 activitiy with an EC50 value of 68.7 μM[1]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1:68.7 μM (EC50) |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 622.6±55.0 °C at 760 mmHg |
| Molecular Formula | C30H44O4 |
| Molecular Weight | 468.668 |
| Flash Point | 344.3±28.0 °C |
| Exact Mass | 468.323975 |
| PSA | 74.60000 |
| LogP | 9.36 |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.550 |
| Hazard Codes | Xi |
|---|