955365-80-7
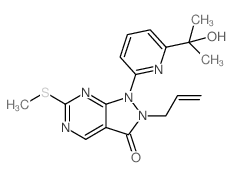
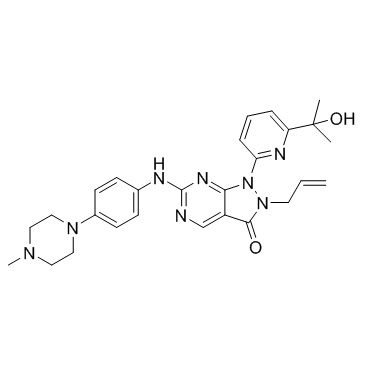
| Name | 1-[6-(2-hydroxypropan-2-yl)pyridin-2-yl]-6-[4-(4-methylpiperazin-1-yl)anilino]-2-prop-2-enylpyrazolo[3,4-d]pyrimidin-3-one |
|---|---|
| Synonyms |
2-allyl-1,8-dimethoxy-9,10-anthraquinone
2-allyl-1-[6-(1-hydroxy-1-methylethyl)pyridin-2-yl]-6-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one 2-Allyl-1-[6-(2-hydroxy-2-propanyl)-2-pyridinyl]-6-{[4-(4-methyl-1-piperazinyl)phenyl]amino}-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one 9,10-Anthracenedione,1,8-dimethoxy-2-(2-propenyl) 2-allyl-1,8-dimethoxyanthracene-9,10-dione MK-1775 MK1775 AZD1775 |
| Description | Adavosertib (AZD-1775; MK-1775) is a potent Wee1 inhibitor with an IC50 of 5.2 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5.2 nM (Wee1) |
| In Vitro | Adavosertib (MK-1775) enhances the cytotoxic effects of 5-FU in p53-deficient human colon cancer cells. Adavosertib (MK-1775) inhibits CDC2 Y15 phosphorylation in cells, abrogates DNA damaged checkpoints induced by 5-FU treatment, and causes premature entry of mitosis determined by induction of Histone H3 phosphorylation[1]. Adavosertib (MK-1775) abrogates the radiation-induced G2 block in p53-defective cells but not in p53 wild-type lines[2]. The combination of gemcitabine with Adavosertib (MK-1775) produces robust anti-tumor activity and remarkably enhances tumor regression response (4.01 fold) compared to gemcitabine treatment in p53-deficient tumors[3]. |
| In Vivo | In vivo, Adavosertib (MK-1775) potentiates the anti-tumor efficacy of 5-FU or its prodrug, capecitabine, at tolerable doses[1]. Adavosertib (MK-1775) (60 mg/kg twice daily, p.o.) enhances H1299 xenograft tumor response to fractionated radiotherapy[2]. Adavosertib (MK-1775) (30 mg/kg. p.o.) regresses tumor growth in PANC198, PANC215 and PANC185 as compared to GEM treated mice[3]. |
| Cell Assay | Total protein is extracted from the cell pellet using a lysis solution containing 50 mM HEPES (pH 7.9), 0.4 mol/L NaCl, and 1 mM EDTA and fortified with 10 µL/mL phosphatase inhibitor cocktail 1, 10 µL/mL phosphatase inhibitor cocktail 2, 10 µL/mL protease inhibitor, and 1% NP-40. Protein concentration of the lysates is determined by the Bio-Rad protein assay. Equal amounts of protein are separated by 12% SDS-PAGE and transferred to an Immobilon membrane. Nonspecific binding sites on the membrane are blocked in 5% nonfat dry milk in Tris (20 mM)-buffered saline (150 mM, pH 7.4) with 0.1% Tween (TBS-T). Protein signals are detected by incubating the membrane in primary antibody in 5% nonfat dry milk overnight at 4°C, followed by a 45-min incubation in the appropriate peroxidase-conjugated secondary antibody. The membrane is then developed by enhanced chemiluminescence with ECL plus Western Blotting Detection Reagents on a Typhoon 9400 scanner. |
| Animal Admin | Tumor xenografts are produced in the leg by im inoculation of 1×106 Calu-6 cells in 10 µL. Irradiation and Adavosertib (MK-1775) treatment are started when tumors reach 8 mm diameter and continue for 5 days. Gamma-rays are delivered locally to the tumor-bearing legs of unanesthetized mice using a small-animal irradiator consisting of two parallel-opposed 137Cs sources, at a dose rate of 5 Gy/min. Tumors are irradiated twice daily separated by 6 h. Adavosertib (MK-1775) is given by gavage in 0.1 mL volumes 1 h before and 2 h after the first daily radiation dose. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 723.8±70.0 °C at 760 mmHg |
| Molecular Formula | C27H32N8O2 |
| Molecular Weight | 500.595 |
| Flash Point | 391.5±35.7 °C |
| Exact Mass | 500.264832 |
| PSA | 104.34000 |
| LogP | 0.50 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.655 |
| Hazard Codes | Xi |
|---|
|
~% 
955365-80-7 |
| Literature: WO2008/133866 A1, ; Page/Page column 29-30 ; WO 2008/133866 A1 |
|
~% 
955365-80-7 |
| Literature: US2007/254892 A1, ; Page/Page column 51 ; US 20070254892 A1 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |