1393477-72-9
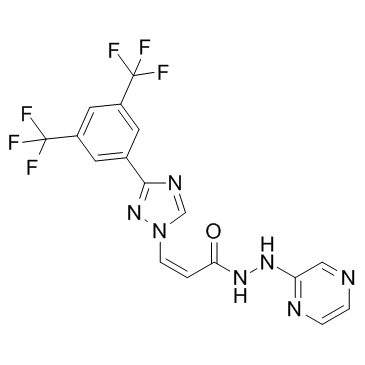
| Name | (Z)-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]-N'-pyrazin-2-ylprop-2-enehydrazide |
|---|---|
| Synonyms |
UNII-31TZ62FO8F
2-Propenoic acid,3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-,2-(2-pyrazinyl)hydrazide,(2Z) (2Z)-3-{3-[3,5-Bis(trifluoromethyl)phenyl]-1H-1,2,4-triazol-1-yl}-N'-(2-pyrazinyl)acrylohydrazide selinexor (Z)-3-(3-(3,5-Bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N'-(pyrazin-2-yl)acrylohydrazide KPT-330 Selinexor (KPT-330) |
| Description | KPT-330, analog of KPT-185, is an orally bioavailable selective CRM1 inhibitor.IC50 value: Target: CRM1in vitro: As the clinical candidate analog of KPT-185, KPT-330 exhibits similar effects on the viability of T-ALL cells and elicits rapid apoptotic response. KPT-330 also reduces cell growth in MOLT-4, Jurkat, HBP-ALL, KOPTK-1, SKW-3, and DND-41 cell lines, with IC50 values of 34-203 nM [1]. in vivo: KPT-330 dramatically suppresses the growth of T-ALL cells (MOLT-4) and AML cells (MV4–11) in vivo, with little toxicity to normal haematopoietic cells [1]. In SCID mice with diffuse human MM bone lesions, KPT-330 inhibits MM-induced bone lysis and prolongs survival. Moreover, KPT-330 directly impairs osteoclastogenesis and bone resorption by blocking RANKL-induced NF-κB and NFATc1, with minimal impact on osteoblasts and BMSCs [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C17H11F6N7O |
| Molecular Weight | 443.306 |
| Exact Mass | 443.092926 |
| PSA | 97.62000 |
| LogP | 3.62 |
| Index of Refraction | 1.594 |
| Storage condition | -20℃ |
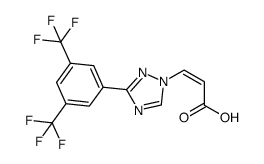
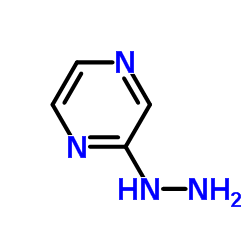
| Precursor 4 | |
|---|---|
| DownStream 0 | |