668270-12-0
| Name | linagliptin |
|---|---|
| Synonyms |
BI-1356-BS
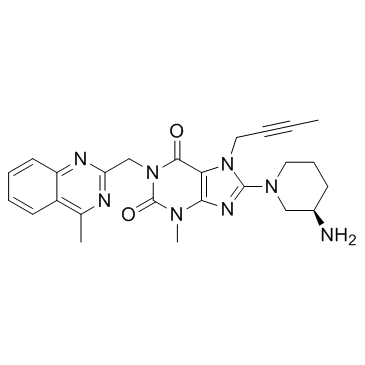
8-[(3R)-3-Amino-1-piperidinyl]-7-(2-butyn-1-yl)-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-3,7-dihydro-1H-purine-2,6-dione Ondero BI-1356 Trajenta Linagliptin 8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione Tradjenta |
| Description | Linagliptin is a highly potent, selective DPP-4 inhibitor with IC50 of 1 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1 nM (DPP-4) |
| In Vitro | Linagliptin inhibits DPP-4 activity in vitro in several independent experiments with IC50 values of 0.4, 0.5, 0.9, and 1.1 nM (mean IC50, approximately 1 nM). Linagliptin inhibits FAP with an IC50 of 89 nM (approximately 90-fold selectivity versus DPP-4)[2]. |
| In Vivo | In male Wistar rats, Beagle dogs, and Rhesus monkeys, xanthine linagliptin proves to be a highly efficacious, long-lasting, and potent DPP-4 inhibitor providing >70% inhibition for >7 h for all three species after oral administration of 1 mg/kg. Single oral administration of linagliptin to db/db mice 45 min prior to an oral glucose tolerance test reduced plasma glucose excursion in a dose-dependent manner from 0.1 mg/kg (15% inhibition) to 1 mg/kg (66% inhibition)[1]. Linagliptin (3 and 10 mg/kg) dose-dependently inhibits the DPP-4 enzyme in plasma within 30 min of administration. Linagliptin (1 mg/kg, p.o.) significantly reduces glucose excursion by approximately 50%[2]. Oral administration of the DPP-4 inhibitor linagliptin (3 mg/kg, p.o.) strongly reduces DPP-4 activity, stabilizes active GLP-1 in chronic wounds, and improves healing in ob/ob mice. At day 10 postwounding, linagliptin-treated ob/ob mice show largely epithelialized wounds characterized by the absence of neutrophils[3]. |
| Kinase Assay | EDTA plasma (20 μL) is diluted with 30 μL of DPP-4 assay buffer (100 mM Tris and 100 mM NaCl, adjusted to pH 7.8 with HCl) and mixed with 50 μL of H-Ala-Pro-7-amido-4-trifluoromethylcoumarin. The 200 mM stock solution in dimethylformamide is diluted 1:1000 with water to yield a final concentration of 100 μM. The plate is incubated at room temperature for 10 min, and fluorescence in the wells is determined by using a Victor 1420 Multilabel Counter at an excitation wavelength of 405 nm and an emission wavelength of 535 nm. For the detection of DPP-4 activity in wound lysates, 100 μg of protein from the respective wound lysates are used instead of 20 μL of plasma. Active GLP-1 is also detected from 100 μg of respective wound tissue samples and analyzed by using the Mouse/Rat Total Active GLP-1 Assay Kit. |
| Cell Assay | A total of 4.0×107 keratinocytes per well are seeded into 24-well plates. After reaching 50% confluence, cells are starved for 24 h with DMEM. Proliferation of cells is assessed by using 1 μCi/mL of [3H]methyl-thymidine in DMEM in the presence of 10% fetal bovine serum and increasing concentrations of linagliptin (3, 30, 300, or 600 nM) for 24 h. Cells are then washed twice with phosphate-buffered saline and incubated in 5% trichloroacetic acid at 4°C for 30 min, and the DNA is solubilized in 0.5mol/LNaOH for 30 min at 37°C. Finally, [3H]thymidine incorporation is determined. |
| Animal Admin | Each experimental group (vehicle or linagliptin treatment) consists of 10 individual ob/ob mice (n=10). Animals are treated orally once a day (8:00 AM) by gastrogavage using vehicle (1% methylcellulose) or linagliptin (3 mg/kg body weight in 1% methylcellulose) beginning 2 days (day−2) before wounding. After wounding, animals are subsequently treated once a day throughout the 10-day healing period. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 661.2±65.0 °C at 760 mmHg |
| Melting Point | 202ºC |
| Molecular Formula | C25H28N8O2 |
| Molecular Weight | 472.542 |
| Flash Point | 353.7±34.3 °C |
| Exact Mass | 472.233521 |
| PSA | 116.86000 |
| LogP | 1.99 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.717 |
| Storage condition | 2-8C |