73604-30-5
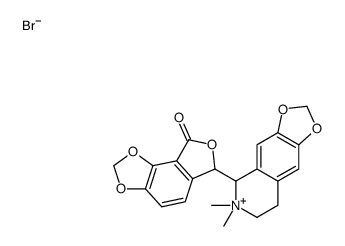
| Name | 6-(6,6-dimethyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-6-ium-5-yl)-6H-furo[3,4-g][1,3]benzodioxol-8-one,bromide |
|---|---|
| Synonyms |
Guvacine hydrochloride
MFCD00055149 |
| Description | (-)-Bicuculline methobromide (l-Bicuculline methobromide) is a potent GABAA receptor antagonist. (-)-Bicuculline methobromide blocks afterhyperpolarizations (AHPs) mediated by Ca2+-activated K+ channels in various types of neurons[1]. |
|---|---|
| Related Catalog | |
| Target |
GABAA[1] |
| In Vivo | (-)-Bicuculline methobromide (0.6 nmol/rat) attenuates the antiallodynic effect of Neurotropin[2]. Animal Model: Rat L5-SNL model[2] Dosage: 0.6 nmol/rat Administration: Intrathecal injection, 5 minutes before administration of Neurotropin (100 NU/kg, i.v.) Result: Attenuated the antiallodynic effect of Neurotropin. |
| References |
| Molecular Formula | C21H20BrNO6 |
|---|---|
| Molecular Weight | 462.29100 |
| Exact Mass | 461.04700 |
| PSA | 63.22000 |
| Safety Phrases | S24/25 |
|---|---|
| RIDADR | UN 1544 6.1/PG 2 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |