Calcium sulfate dihydrate
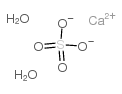
Calcium sulfate dihydrate structure
|
Common Name | Calcium sulfate dihydrate | ||
|---|---|---|---|---|
| CAS Number | 10101-41-4 | Molecular Weight | 172.17100 | |
| Density | 2.32 | Boiling Point | 163°C -2H₂O | |
| Molecular Formula | CaH4O6S | Melting Point | 128°C -1.5H₂O | |
| MSDS | Chinese USA | Flash Point | 163°C -2H₂O | |
| Name | calcium sulfate dihydrate |
|---|---|
| Synonym | More Synonyms |
| Density | 2.32 |
|---|---|
| Boiling Point | 163°C -2H₂O |
| Melting Point | 128°C -1.5H₂O |
| Molecular Formula | CaH4O6S |
| Molecular Weight | 172.17100 |
| Flash Point | 163°C -2H₂O |
| Exact Mass | 171.93500 |
| PSA | 107.10000 |
| Vapour Pressure | 3.35E-05mmHg at 25°C |
| Index of Refraction | 1.5248 |
| InChIKey | PASHVRUKOFIRIK-UHFFFAOYSA-L |
| SMILES | O.O.O=S(=O)([O-])[O-].[Ca+2] |
| Stability | Stable. Incompatible with aluminium, strong acids. |
| Water Solubility | 2 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | EW4150000 |
| HS Code | 28332990 |
| HS Code | 28332990 |
|---|
|
The effect of denture design and fixatives on the retention of mandibular complete dentures tested on a novel in-vitro edentulous model.
Eur. J. Prosthodont. Restor. Dent. 21(2) , 64-74, (2013) The aim of this study was to evaluate the effect of the design (extension and adaptation) of a mandibular complete acrylic denture and the use of denture adhesives using a novel in-vitro edentulous mo... |
|
|
Sealing ability of various restorative materials as coronal barriers between endodontic appointments.
J. Contemp. Dent. Pract. 14(1) , 47-50, (2013) To evaluate and compare the microleakage of various restorative materials used as coronal barriers between endodontic appointments.Eighty extracted human permanent posterior teeth were prepared for st... |
|
|
Elemental geochemistry of sedimentary rocks at Yellowknife Bay, Gale crater, Mars.
Science 343(6169) , 1244734, (2014) Sedimentary rocks examined by the Curiosity rover at Yellowknife Bay, Mars, were derived from sources that evolved from an approximately average martian crustal composition to one influenced by alkali... |
| alabaster |
| gypse |
| lightspar |
| annaline |
| EINECS 231-900-3 |
| MFCD00149625 |
| TERRA ALBA |
| satinite |
| SATIN SPAR |
| GYPSUM |
| Calcium sulfate dihydrate |

