Astat
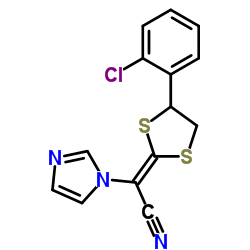
Astat structure
|
Common Name | Astat | ||
|---|---|---|---|---|
| CAS Number | 101530-10-3 | Molecular Weight | 319.832 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 477.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C14H10ClN3S2 | Melting Point | 141.50C | |
| MSDS | Chinese USA | Flash Point | 242.6±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AstatLanoconazole is a potent and orally active imidazole antifungal agent, shows a broad spectrum of activity against fungi in vitro and in vivo[1]. Lanoconazole interferes with ergosterol biosynthesis by inhibiting sterol 14-alpha demethylase and blocking fungal membrane ergosterol biosynthesis. Lanoconazole can be used for the investigation of dermatophytosis and onychomycosis[1][2]. |
| Name | Lanoconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Lanoconazole is a potent and orally active imidazole antifungal agent, shows a broad spectrum of activity against fungi in vitro and in vivo[1]. Lanoconazole interferes with ergosterol biosynthesis by inhibiting sterol 14-alpha demethylase and blocking fungal membrane ergosterol biosynthesis. Lanoconazole can be used for the investigation of dermatophytosis and onychomycosis[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: antifungal[1] |
| In Vivo | Lanoconazole (treatment for ear; 0.3%-3%; 6 days) dose‐dependently suppressesTPA-induced irritant dermatitis, suppresses the production of neutrophil chemotactic factors such as keratinocyte‐derived chemokine and macrophage inflammatory protein‐2, and inhibited neutrophil infiltration to the inflammation site[2]. Lanoconazole (oral administration; 3, 10 or 30 mg/kg; once a day; 3 weeks) significantly inhibits C. neoformans compared with the saline control in normal mice. In addtion, it significantly reduces the growth of C. neoformans in the lungs and brains of MAIDS mice[3]. Animal Model: BALB/c mice[2] Dosage: 0.3%-3% dosage Administration: Treatment for ear Result: Exhibited an inhibition effect of LCZ on ear swelling induced by topical application of TPA in mice. Animal Model: Four week old C57BL/6 mice infected intraperitoneally with LP-BM5 murine leukaemia virus[3] Dosage: 3, 10 or 30 mg/kg Administration: Oral adminstration Result: Inhibited C. neoformans growth in both normal and C. neoformans -induced encephalitis MAIDS mice . |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 477.6±55.0 °C at 760 mmHg |
| Melting Point | 141.50C |
| Molecular Formula | C14H10ClN3S2 |
| Molecular Weight | 319.832 |
| Flash Point | 242.6±31.5 °C |
| Exact Mass | 319.000458 |
| PSA | 92.21000 |
| LogP | 3.38 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.725 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| RTECS | NI3393500 |
|
Allergic contact dermatitis due to both lanoconazole and neticonazole ointments.
Contact Dermatitis 44(1) , 48-9, (2001)
|
|
|
Allergic contact dermatitis from diethyl sebacate in lanoconazole cream.
Contact Dermatitis 43(4) , 233-4, (2000)
|
|
|
Allergic contact dermatitis from lanoconazole.
Contact Dermatitis 35(1) , 63, (1996)
|
| (2Z)-[4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene](1H-imidazol-1-yl)ethanenitrile |
| [4-(2-Chloro-phenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-yl-acetonitrile |
| (E)-[4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene]-1-imidazol-1-yl acetonitrile |
| (E)-(±)-a-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene]-1H-imidazole-1-acetonitrile |
| latoconazole |
| nnd-318 |
| (+-)-(E)-[4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene]-1-imidazolylacetonitrile |
| MFCD00865590 |
| (2Z)-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene](1H-imidazol-1-yl)acetonitrile |
| 2-(1-imidazolyl)-2-[4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene]acetonitrile |
| [4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene](1H-imidazol-1-yl)acetonitrile |
| 2-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-yl-acetonitrile |
| tjn-318 |
| 1H-Imidazole-1-acetonitrile, α-[4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene]-, (αZ)- |
| (4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene)-1-imidazolylacetonitrile |
| Astat |
| itrile |