Bakuchiol
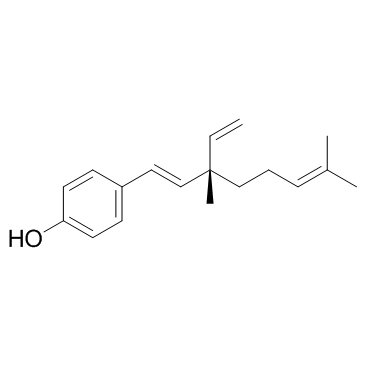
Bakuchiol structure
|
Common Name | Bakuchiol | ||
|---|---|---|---|---|
| CAS Number | 10309-37-2 | Molecular Weight | 256.383 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 391.4±21.0 °C at 760 mmHg | |
| Molecular Formula | C18H24O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 176.6±11.7 °C | |
Use of BakuchiolBakuchiol is a phytoestrogen isolated from the seeds of Psoralea corylifolia L; has anti-tumor effects.IC50 value:Target:in vitro: Bakuchiol reduced mitochondrial membrane potential (Psim) of cells in a concentration- and time-dependent manner, showing a more potent effect than that of resveratrol. S phase arrest, caspase 9/3 activaton, p53 and Bax up-regulation, as well as Bcl-2 down-regulation were observed in bakuchiol-treated A549 cells [1]. UGT2B7 was inhibited by the strongest intensity. The noncompetitive inhibition was demonstrated by the results obtained from Dixon plot and Lineweaver-Burk plot. The Ki value was calculated to be 10.7 μM [2]. Bakuchiol was found to be naturally occurring potent inhibitors of hCE2, with low Ki values ranging from 0.62μM to 3.89μM [3]. After exposure to bakuchiol at 0.25-fold, 0.5-fold and 1-fold of minimum inhibitory concentration (MIC) (3.91 μg/ml) for 24h, the fungal conidia of T. mentagrophytes demonstrated a significant dose-dependent increase in membrane permeability. Moreover, bakuchiol at 1-fold MIC elicited a 187% elevation in reactive oxygen species (ROS) level in fungal cells after a 3-h incubation [4].in vivo: In combination with the reported concentration after an intravenous administration of bakuchiol (15 mg/kg) in rats, the high risk of in vivo inhibition of bakuchiol towards UGT2B7-catalyzed metabolism of drugs was indicated [2]. In a guinea pig model of tinea pedis, bakuchiol at 1%, 5% or 10% (w/w) concentration in aqueous cream could significantly reduce the fungal burden of infected feet (p<0.01-0.05) [4]. |
| Name | 4-(3,7-Dimethyl-3-vinylocta-1,6-dien-1-yl)phenol |
|---|---|
| Synonym | More Synonyms |
| Description | Bakuchiol is a phytoestrogen isolated from the seeds of Psoralea corylifolia L; has anti-tumor effects.IC50 value:Target:in vitro: Bakuchiol reduced mitochondrial membrane potential (Psim) of cells in a concentration- and time-dependent manner, showing a more potent effect than that of resveratrol. S phase arrest, caspase 9/3 activaton, p53 and Bax up-regulation, as well as Bcl-2 down-regulation were observed in bakuchiol-treated A549 cells [1]. UGT2B7 was inhibited by the strongest intensity. The noncompetitive inhibition was demonstrated by the results obtained from Dixon plot and Lineweaver-Burk plot. The Ki value was calculated to be 10.7 μM [2]. Bakuchiol was found to be naturally occurring potent inhibitors of hCE2, with low Ki values ranging from 0.62μM to 3.89μM [3]. After exposure to bakuchiol at 0.25-fold, 0.5-fold and 1-fold of minimum inhibitory concentration (MIC) (3.91 μg/ml) for 24h, the fungal conidia of T. mentagrophytes demonstrated a significant dose-dependent increase in membrane permeability. Moreover, bakuchiol at 1-fold MIC elicited a 187% elevation in reactive oxygen species (ROS) level in fungal cells after a 3-h incubation [4].in vivo: In combination with the reported concentration after an intravenous administration of bakuchiol (15 mg/kg) in rats, the high risk of in vivo inhibition of bakuchiol towards UGT2B7-catalyzed metabolism of drugs was indicated [2]. In a guinea pig model of tinea pedis, bakuchiol at 1%, 5% or 10% (w/w) concentration in aqueous cream could significantly reduce the fungal burden of infected feet (p<0.01-0.05) [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 391.4±21.0 °C at 760 mmHg |
| Molecular Formula | C18H24O |
| Molecular Weight | 256.383 |
| Flash Point | 176.6±11.7 °C |
| Exact Mass | 256.182709 |
| PSA | 20.23000 |
| LogP | 6.40 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.555 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | F+ |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | SL3785000 |
| HS Code | 2907199090 |
| HS Code | 2907199090 |
|---|---|
| Summary | 2907199090 other monophenols VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Bakuchiol derivatives as novel and potent cytotoxic agents: a report.
Eur. J. Med. Chem. 49C , 55-67, (2012) A library of 28 compounds comprising of acyl, amino, halo, nitro, styryl and cyclized derivatives of bakuchiol have been evaluated against a panel of eight human cancer cell lines. Bioevaluation studi... |
|
|
Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation.
Planta Med. 78(9) , 903-6, (2012) Inhibiting interleukin-6 (IL-6) has been postulated as an effective therapy in the pathogenesis of several inflammatory diseases. In this study, seven flavonoids were isolated from the methanol extrac... |
|
|
Chemical fingerprint and quantitative analysis of fructus psoraleae by high-performance liquid chromatography.
J. Sep. Sci. 30(6) , 813-8, (2007) Fructus Psoraleae, a widely used traditional Chinese medicine, is well known as a health supplement ingredient. In our study, an improved and comprehensive HPLC fingerprint of Fructus Psoraleae was es... |
| P-(3,7-dimethyl-3-vinylocta-trans-1,6-dimethyl) phenol |
| Phenol, 4-[(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]- |
| BACTRIS GASIPAES FRUIT JUICE |
| 4-[(1E,3S)-3-ethenyl-3,7-dimethylocta-1,6-dien-1-yl]phenol |
| Backuchiol |
| Bakuchiol |
| 4-[(1E,3S)-3,7-Dimethyl-3-vinyl-1,6-octadien-1-yl]phenol |
| Drupanol |
| 4-[(1E,3S)-3,7-Dimethyl-3-vinylocta-1,6-dien-1-yl]phenol |
| (S)-bakuchiol |
| UP 256 |
| Phenol, 4-[(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadienyl]- |

