Ferric nitrate
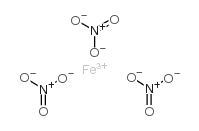
Ferric nitrate structure
|
Common Name | Ferric nitrate | ||
|---|---|---|---|---|
| CAS Number | 10421-48-4 | Molecular Weight | 241.86000 | |
| Density | 1.684 | Boiling Point | 83ºC at 760 mmHg | |
| Molecular Formula | FeN3O9 | Melting Point | 47.2ºC | |
| MSDS | Chinese | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | iron(3+),trinitrate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.684 |
|---|---|
| Boiling Point | 83ºC at 760 mmHg |
| Melting Point | 47.2ºC |
| Molecular Formula | FeN3O9 |
| Molecular Weight | 241.86000 |
| Exact Mass | 241.89800 |
| PSA | 206.64000 |
| LogP | 0.84980 |
| Vapour Pressure | 49.8mmHg at 25°C |
Synonym:Ferric nitrate; Iron trinitrate; Iron nitrate Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 36/38 8 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Irritating to eyes and skin. Contact with combustible material may cause fire.Hygroscopic.Moisture sensitive.The toxicological properties of this material have not been fully investigated.Mutagen.Light sensitive. Potential Health Effects Eye: Causes eye irritation. Skin: Causes skin irritation. Ingestion: Causes gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Inhalation: Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Chronic: No information found. Section 4 - FIRST AID MEASURES Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower lids. Get medical aid. Skin: Immediately flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Strong oxidizer. Contact with combustible materials may cause a fire. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Extinguishing Media: Substance is nonflammable; use agent most appropriate to extinguish surrounding fire. Autoignition Temperature: Not applicable. Flash Point: Not applicable. NFPA Rating: Not published. Explosion Limits, Lower: Not available. Upper: Not available. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Reduce airborne dust and prevent scattering by moistening with water. Clean up spills immediately, observing precautions in the Protective Equipment section. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Use only in a well ventilated area. Avoid contact with skin and eyes. Avoid contact with clothing and other combustible materials. Avoid ingestion and inhalation. Storage: Do not store near combustible materials. Store in a cool, dry place. Do not store in direct sunlight. Keep container closed when not in use. Store in a tightly closed container. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate general or local, explosion-proof ventilation to keep airborne levels to acceptable levels. Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29CFR 1910.134. Always use a NIOSH-approved respirator when necessary. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Appearance: light purple Odor: None reported. pH: Not available. Vapor Pressure: Not available. Vapor Density: Not available. Evaporation Rate: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 47 deg C Decomposition Temperature: Not available. Solubility: Soluble in water. Specific Gravity/Density: 1.68 Molecular Formula: N3O9Fe Molecular Weight: 241.8617 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable at room temperature in closed containers under normal storage and handling conditions. Conditions to Avoid: Incompatible materials, combustible materials, exposure to moist air or water. Incompatibilities with Other Materials: Strong oxidizers. May discolor upon exposure to light. Hazardous Decomposition Products: Oxides of nitrogen, irritating and toxic fumes and gases. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 10421-48-4: QU8915000 LD50/LC50: Not available. Carcinogenicity: Ferric nitrate - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA. See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION For further information, contact Fisher Scientific. Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION CDG/CPL Shipping Name: FERRIC NITRATE Hazard Class: 5.1 UN Number: 1466 Packing Group: III IMO No information available. IATA No information available. RID/ADR No information available. Canadian TDG No information available. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XI O Risk Phrases: R 36/38 Irritating to eyes and skin. R 8 Contact with combustible material may cause fire. Safety Phrases: S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 28 After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer). WGK (Water Danger/Protection) CAS# 10421-48-4: 1 Canada CAS# 10421-48-4 is listed on Canada's DSL/NDSL List. WHMIS: Not available. CAS# 10421-48-4 is not listed on Canada's Ingredient Disclosure List. Exposure Limits CAS# 10421-48-4:. OEL-DENMARK:TWA 1 mg(Fe)/m3 JANUARY 1993. OEL-FINL AND:TWA 1 mg(Fe)/m3 JANUARY 1993. OEL-THE NETHERLANDS:TWA 1 mg(Fe)/m3 JANUARY 1993. OEL-SWITZERLAND:TWA 1 mg(Fe)/m3 JANUARY 1993. OEL-UNI TED KINGDOM:TWA 1 mg(Fe)/m3;STEL 2 mg(Fe)/m3 JANUARY 1993. OEL IN BUL GARIA, COLOMBIA, JORDAN, KOREA check ACGIH TLV. OEL IN NEW ZEALAND, SI NGAPORE, VIETNAM check ACGI TLV US FEDERAL TSCA CAS# 10421-48-4 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | R36/38 |
| Safety Phrases | 26 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 1 |
| Packaging Group | III |
| Hazard Class | 5.1 |
| HS Code | 2834299090 |
| HS Code | 2834299090 |
|---|
|
A pharmaceutical study of doxorubicin-loaded PEGylated nanoparticles for magnetic drug targeting.
Int. J. Pharm. 423(1) , 16-25, (2012) One of the new strategies to improve cancer chemotherapy is based on new drug delivery systems, like the polyethylene glycol-coated superparamagnetic iron oxide nanoparticles (PEG-SPION, thereafter ca... |
|
|
An unprecedented Fe(36) phosphonate cage.
Inorg. Chem. 52(4) , 1670-2, (2013) The reaction of 2-pyridylphosphonic acid (LH(2)) with iron(II) perchlorate and iron(III) nitrate afforded an interconnected, double-layered, cationic iron cage, [{Fe(36)L(44)(H(2)O)(48)}](20+) (1a), t... |
|
|
Stress and the periodontal diseases: growth responses of periodontal bacteria to Escherichia coli stress-associated autoinducer and exogenous Fe.
Oral Microbiol. Immunol. 20(3) , 147-53, (2005) Psychological stress is known to increase the circulating levels of the catecholamine hormones noradrenaline and adrenaline, which have been shown to influence the growth of a large number of bacteria... |
| UN1466 |
| Iron nitrate |
| Iron trinitrate |
| Nitric acid,iron(3+) salt |
| EINECS 233-899-5 |
| UNII-N8H8402XOB |
| Iron(III) nitrate,anhydrous |
| Iron nitrate (Fe(NO3)3) |
| MFCD00011003 |