Potassium phthalimide
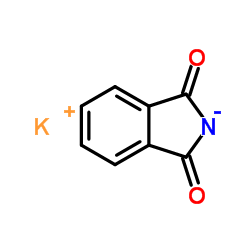
Potassium phthalimide structure
|
Common Name | Potassium phthalimide | ||
|---|---|---|---|---|
| CAS Number | 1074-82-4 | Molecular Weight | 185.221 | |
| Density | 1.367g/cm3 | Boiling Point | 359ºC at 760 mmHg | |
| Molecular Formula | C8H4KNO2 | Melting Point | >300°C | |
| MSDS | USA | Flash Point | 170.9ºC | |
| Name | Potassium Phthalimide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.367g/cm3 |
|---|---|
| Boiling Point | 359ºC at 760 mmHg |
| Melting Point | >300°C |
| Molecular Formula | C8H4KNO2 |
| Molecular Weight | 185.221 |
| Flash Point | 170.9ºC |
| Exact Mass | 184.987915 |
| PSA | 34.14000 |
| LogP | 0.68480 |
| InChIKey | FYRHIOVKTDQVFC-UHFFFAOYSA-M |
| SMILES | O=C1[N-]C(=O)c2ccccc21.[K+] |
| Storage condition | Store at RT. |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2925190090 |
|
~89% 
Potassium phtha... CAS#:1074-82-4 |
| Literature: Journal of the American Chemical Society, , vol. 133, # 41 p. 16410 - 16413 |
|
~% 
Potassium phtha... CAS#:1074-82-4 |
| Literature: Chemische Berichte, , vol. 21, p. 2686 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2925190090 |
|---|---|
| Summary | 2925190090 other imides and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Synthesis and anti-angiogenic activity of benzothiazole, benzimidazole containing phthalimide derivatives.
Bioorg. Med. Chem. Lett. 23(1) , 287-90, (2013) Benzothiazole and benzimidazole containing phthalimide derivatives (NK037, NK041, NK042, NK0139A and NK0148) have been synthesized and their anti-angiogenic activity was evaluated using ex vivo egg yo... |
|
|
Azolyacetones as precursors to indoles and naphthofurans facilitated by microwave irradiation with simultaneous cooling.
Molecules 14(8) , 2976-84, (2009) Phthalimide reacted with phenacyl bromide under microwave irradiation to yield phenacyl isoindolidene-1,3-dione (3b), while 3a reacted with phenylhydrazine to yield the phenylhydrazone 4 that was read... |
|
|
A review of methods for the analysis of orphan and difficult pesticides: glyphosate, glufosinate, quaternary ammonium and phenoxy acid herbicides, and dithiocarbamate and phthalimide fungicides.
J. AOAC Int. 97(4) , 965-77, (2014) This article reviews the chromatography/MS methodologies for analysis of pesticide residues of orphan and difficult chemical classes in a variety of sample matrixes including water, urine, blood, and ... |
| Potassium phthalimide |
| 1H-Isoindole-1,3(2H)-dione, potassium salt (1:1) |
| Phthalimide potassium salt |
| EINECS 214-046-6 |
| Potassium 1,3-dioxo-1,3-dihydroisoindol-2-ide |
| MFCD00005887 |
| Potassium 1,3-dioxoisoindolin-2-ide |
| phthalimide potassium |
| 1,3-Dihydro-1,3-dioxoisoindole potassium salt |
| Phthalimide,potassium derivative |

 CAS#:10513-98-1
CAS#:10513-98-1 CAS#:24566-79-8
CAS#:24566-79-8 CAS#:10513-96-9
CAS#:10513-96-9 CAS#:110859-48-8
CAS#:110859-48-8 CAS#:105260-10-4
CAS#:105260-10-4![2-[3-(4-chlorophenyl)-3-oxopropyl]isoindole-1,3-dione structure](https://image.chemsrc.com/caspic/099/112031-92-2.png) CAS#:112031-92-2
CAS#:112031-92-2 CAS#:111992-61-1
CAS#:111992-61-1 CAS#:105264-63-9
CAS#:105264-63-9 CAS#:111853-59-9
CAS#:111853-59-9 CAS#:41306-64-3
CAS#:41306-64-3
