alpha-Terthiophene
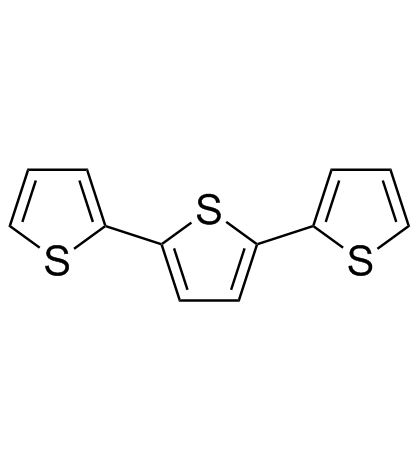
alpha-Terthiophene structure
|
Common Name | alpha-Terthiophene | ||
|---|---|---|---|---|
| CAS Number | 1081-34-1 | Molecular Weight | 248.387 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 361.3±32.0 °C at 760 mmHg | |
| Molecular Formula | C12H8S3 | Melting Point | 93-95 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 128.4±11.3 °C | |
Use of alpha-Terthiophene2,2':5',2''-Terthiophene (α-Terthiophene) is an oligomer of the heterocycle thiophene. 2,2':5',2''-Terthiophene has been employed as building block for the organic semi-conductor polythiophene. |
| Name | 2,2':5',2''-terthiophene |
|---|---|
| Synonym | More Synonyms |
| Description | 2,2':5',2''-Terthiophene (α-Terthiophene) is an oligomer of the heterocycle thiophene. 2,2':5',2''-Terthiophene has been employed as building block for the organic semi-conductor polythiophene. |
|---|---|
| Related Catalog | |
| In Vitro | In the most common isomer of Terthiophene, two thienyl groups are connected via their 2 positions to a central thiophene, also at the carbon atoms flanking the sulfur. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 361.3±32.0 °C at 760 mmHg |
| Melting Point | 93-95 °C(lit.) |
| Molecular Formula | C12H8S3 |
| Molecular Weight | 248.387 |
| Flash Point | 128.4±11.3 °C |
| Exact Mass | 247.978806 |
| PSA | 84.72000 |
| LogP | 5.56 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.672 |
| InChIKey | KXSFECAJUBPPFE-UHFFFAOYSA-N |
| SMILES | c1csc(-c2ccc(-c3cccs3)s2)c1 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F: Flammable; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | WZ9717750 |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Length-dependent conductance of oligothiophenes.
J. Am. Chem. Soc. 136(29) , 10486-92, (2014) We have measured the single-molecule conductance of a family of oligothiophenes comprising 1-6 thiophene moieties terminated with methyl-sulfide linkers using the scanning tunneling microscope-based b... |
|
|
Radical-scavenging activity of melatonin, either alone or in combination with vitamin E, ascorbate or 2-mercaptoethanol as co-antioxidants, using the induction period method.
In Vivo 25(1) , 49-53, (2011) Melatonin shows antioxidant/prooxidant activity but its mechanism of action remains unknown.The radical-scavenging activity of melatonin and various melatonin/co-antioxidant mixtures in a 1:1 molar ra... |
|
|
Synthesis and evaluation of new spacers for use as dsDNA end-caps.
Bioconjug. Chem. 21(8) , 1545-53, (2010) A series of aliphatic and aromatic spacer molecules designed to cap the ends of DNA duplexes have been synthesized. The spacers were converted into dimethoxytrityl-protected phosphoramidites as syntho... |
| Terthiophene |
| 2,2':5',2"-Terthiophene |
| 2,2':5',2''-Terthiophene |
| 2,2':5'2''-Terthiophene |
| 2,2:5,2-Terthiophene |
| a-Terthienyl |
| MFCD00012167 |
| 5-(2-Thienyl)-2,2'-bithiophene |
| 2,5-Di(2-thienyl)thiophene |
| α-Terthienyl |
| 2,5-dithiophen-2-ylthiophene |
| [2,2';5',2'']Terthiophene |

