Magainin 1
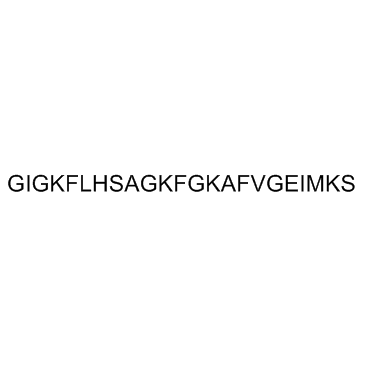
Magainin 1 structure
|
Common Name | Magainin 1 | ||
|---|---|---|---|---|
| CAS Number | 108433-99-4 | Molecular Weight | 2409.85000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C112H177N29O28S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Magainin 1Magainin 1 is an antimicrobial peptide discovered in the skin of Xenopus laevis. |
| Name | magainin i |
|---|---|
| Synonym | More Synonyms |
| Description | Magainin 1 is an antimicrobial peptide discovered in the skin of Xenopus laevis. |
|---|---|
| Related Catalog | |
| In Vitro | Magainin 1 kill bacteria by permeabilizing the cell membranes without exhibiting significant toxicity against mammalian cells. The main target of the peptide is considered to be the lipid matrix of the membranes[1]. Magainin 1 and 2 have a similar amino-acid sequence. Magainin 2 has higher antimicrobial activity than magainin 1[2]. Magainin 1 interacts with acidic lipids through electrostatic interactions followed by hydrophobic interactions to form an amphiphilic helix, inducing the leakage. Magainin 1 induces the leakage of calcein specifically out of negatively-charged vesicles. The peptide binds to bovine brain phosphatidylserine sonicated vesicles according to the Langmuir isotherm with a binding constant of 3.8×105 M-1 and a binding-site number of 0.10 per lipid molecule[3]. Magainin 2 displays antibiotic activity against numerous Gram-negative and Gram-positive bacteria. A similar spectrum of activity is seen on assay of magainin 1[4]. |
| References |
| Molecular Formula | C112H177N29O28S |
|---|---|
| Molecular Weight | 2409.85000 |
| Exact Mass | 2408.30000 |
| PSA | 939.34000 |
| LogP | 4.96870 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T+ |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides.
Antimicrob. Agents Chemother. 58(7) , 4113-22, (2014) The seriousness of microbial resistance combined with the lack of new antimicrobials has increased interest in the development of antimicrobial peptides (AMPs) as novel therapeutics. In this study, we... |
|
|
In vitro efficacy of a synthetic all-d antimicrobial peptide against clinically isolated drug-resistant strains.
Int. J. Antimicrob. Agents 35(2) , 208-9, (2010)
|
|
|
NMR structural studies of membrane proteins.
Curr. Opin. Struct. Biol. 8(5) , 640-8, (1998) The three-dimensional structures of membrane proteins are essential for understanding their functions, interactions and architectures. Their requirement for lipids has hampered structure determination... |
| GLY-ILE-GLY-LYS-PHE-LEU-HIS-SER-ALA-GLY-LYS-PHE-GLY-LYS-ALA-PHE-VAL-GLY-GLU-ILE-MET-LYS-SER |
| Gly-L-Ile-Gly-L-Lys-L-Phe-L-Leu-L-His-L-Ser-L-Ala-Gly-L-Lys-L-Phe-Gly-L-Lys-L-Ala-L-Phe-L-Val-Gly-L-Glu-L-Ile-L-Met-L-Lys-L-Ser-OH |
| MAGAININ 1 (FROG,XENOPUS LAEVIS) |
| H-GLY-ILE-GLY-LYS-PHE-LEU-HIS-SER-ALA-GLY-LYS-PHE-GLY-LYS-ALA-PHE-VAL-GLY-GLU-ILE-MET-LYS-SER-OH |
| GIGKFLHSAGKFGKAFVGEIMKS |
| Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Gly-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Lys-Ser-OH |
| MAGAININ 1 |

