tepotinib
Modify Date: 2024-01-05 11:15:30
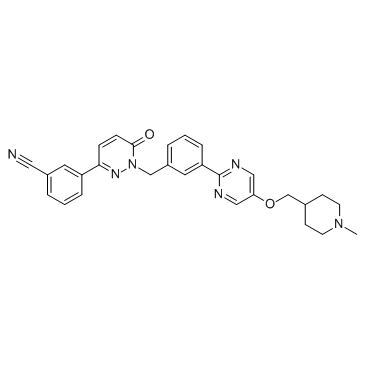
tepotinib structure
|
Common Name | tepotinib | ||
|---|---|---|---|---|
| CAS Number | 1100598-32-0 | Molecular Weight | 492.572 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 626.5±65.0 °C at 760 mmHg | |
| Molecular Formula | C29H28N6O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 332.7±34.3 °C | |
Use of tepotinibEMD 1214063 is a potent and selective c-Met inhibitor with IC50 of 4 nM, >200-fold selective for c-Met than IRAK4, TrkA, Axl, IRAK1, and Mer. IC50 Value: 4 nM [1]Target: c-Metin vitro: EMD 1214063 inhibits HGF-induced c-Met phosphorylation in A549 cells with IC50 of 6 nM. Treatment with EMD 1214063 induces a marked reduction of c-Met–constitutive phosphorylation in EBC-1 cells with IC50 of 9 nM. EMD 1214063 effectively blocka phosphorylation of the major downstream effectors of the c-Met enzyme, such as Grb2, Gab1, Sos, PLCγ, and phosphoinositide 3-kinase, in EBC-1, MKN-45, and Hs746T cells in the range of 1 to 10 nM. EMD 1214063 considerably inhibits the viability of MKN-45 cells with IC50 of less than 1 nM. Treatment with EMD 1214063 (as low as 0.1 nM) inhibits HGF-induced NCI-H441 cell migration, whereas concentrations of 100 nM to 1 μM almost completely prevents it.in vivo: EMD 1214063 treatment, at doses of 10 mg/kg or more, results in more than 90% inhibition of c-Met phosphorylation in Hs746T xenograft tumor for a period of at least 72 hours. EMD 1214063 induces more than 50% reduction of cyclin D1 expression, which persists after 96 hours upon treatment with doses of 100 mg/kg. A transient induction of p27 and cleaved caspase-3 are also observed upon treatment with EMD 1214063. EMD 1214063 (15 mg/kg, daily) treatment induces complete regression of gastric carcinoma xenografts Hs746T, in which c-Met is amplified, overexpressed, and activated in a ligand-independent fashion. |
| Name | 3-[1-[[3-[5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl]phenyl]methyl]-6-oxopyridazin-3-yl]benzonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | EMD 1214063 is a potent and selective c-Met inhibitor with IC50 of 4 nM, >200-fold selective for c-Met than IRAK4, TrkA, Axl, IRAK1, and Mer. IC50 Value: 4 nM [1]Target: c-Metin vitro: EMD 1214063 inhibits HGF-induced c-Met phosphorylation in A549 cells with IC50 of 6 nM. Treatment with EMD 1214063 induces a marked reduction of c-Met–constitutive phosphorylation in EBC-1 cells with IC50 of 9 nM. EMD 1214063 effectively blocka phosphorylation of the major downstream effectors of the c-Met enzyme, such as Grb2, Gab1, Sos, PLCγ, and phosphoinositide 3-kinase, in EBC-1, MKN-45, and Hs746T cells in the range of 1 to 10 nM. EMD 1214063 considerably inhibits the viability of MKN-45 cells with IC50 of less than 1 nM. Treatment with EMD 1214063 (as low as 0.1 nM) inhibits HGF-induced NCI-H441 cell migration, whereas concentrations of 100 nM to 1 μM almost completely prevents it.in vivo: EMD 1214063 treatment, at doses of 10 mg/kg or more, results in more than 90% inhibition of c-Met phosphorylation in Hs746T xenograft tumor for a period of at least 72 hours. EMD 1214063 induces more than 50% reduction of cyclin D1 expression, which persists after 96 hours upon treatment with doses of 100 mg/kg. A transient induction of p27 and cleaved caspase-3 are also observed upon treatment with EMD 1214063. EMD 1214063 (15 mg/kg, daily) treatment induces complete regression of gastric carcinoma xenografts Hs746T, in which c-Met is amplified, overexpressed, and activated in a ligand-independent fashion. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 626.5±65.0 °C at 760 mmHg |
| Molecular Formula | C29H28N6O2 |
| Molecular Weight | 492.572 |
| Flash Point | 332.7±34.3 °C |
| Exact Mass | 492.227386 |
| PSA | 96.93000 |
| LogP | 2.72 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.660 |
| Storage condition | -20℃ |
| Hazard Codes | N |
|---|
|
~% 
tepotinib CAS#:1100598-32-0 |
| Literature: MERCK PATENT GESELLSCHAFT Patent: US2010/197690 A1, 2010 ; Location in patent: Page/Page column 18 ; |
|
~% 
tepotinib CAS#:1100598-32-0 |
| Literature: MERCK PATENT GMBH; BECKER, Axel; KUEHN, Clemens; SAAL, Christoph; SCHADT, Oliver; DORSCH, Dieter; BOKEL, Heinz-Hermann; STIEBER, Frank; DONINI, Christina Patent: WO2010/78897 A1, 2010 ; Location in patent: Page/Page column 18 ; |
| Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]- |
| tepotinib |
| 3-(1-(3-(5-(1-methylpiperidin-4-ylmethoxy)pyrimidin-2-yl)benzyl)-1,6-dihydro-6-oxopyridazin-3-yl)benzonitrile |
| 3-(1-{3-[5-(1-methylpiperidin-4-ylmethoxy)pyrimidin-2-yl]benzyl}-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile |
| 3-[1-(3-{5-[(1-Methyl-4-piperidinyl)methoxy]-2-pyrimidinyl}benzyl)-6-oxo-1,6-dihydro-3-pyridazinyl]benzonitrile |
| EMD 1214063 |
| EMD1214063 |
| EMD-1214063 |
![BenzeneMethanol, 3-[5-[(1-Methyl-4-piperidinyl)Methoxy]-2-pyrimidinyl]- structure](https://image.chemsrc.com/caspic/416/1100598-48-8.png)

![4-(2-{3-[3-(3-Cyano-phenyl)-6-oxo-6H-pyridazin-1-ylmethyl]-phenyl}-pyrimidin-5-yloxymethyl)-piperidine-1-carbonic acid tert-butyl ester structure](https://image.chemsrc.com/caspic/456/1103506-80-4.png)