Squalene

Squalene structure
|
Common Name | Squalene | ||
|---|---|---|---|---|
| CAS Number | 111-02-4 | Molecular Weight | 410.718 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 429.3±0.0 °C at 760 mmHg | |
| Molecular Formula | C30H50 | Melting Point | −75 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 254.1±22.2 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of SqualeneSqualene is an intermediate product in the synthesis of cholesterol, and shows several pharmacological properties such as hypolipidemic, hepatoprotective, cardioprotective, antioxidant, and antitoxicant activity. |
| Name | squalene |
|---|---|
| Synonym | More Synonyms |
| Description | Squalene is an intermediate product in the synthesis of cholesterol, and shows several pharmacological properties such as hypolipidemic, hepatoprotective, cardioprotective, antioxidant, and antitoxicant activity. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 0.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 429.3±0.0 °C at 760 mmHg |
| Melting Point | −75 °C(lit.) |
| Molecular Formula | C30H50 |
| Molecular Weight | 410.718 |
| Flash Point | 254.1±22.2 °C |
| Exact Mass | 410.391266 |
| LogP | 13.09 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.492 |
| Storage condition | 2-8°C |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H304 |
| Precautionary Statements | P301 + P310-P331 |
| Personal Protective Equipment | Eyeshields;Gloves |
| Hazard Codes | F |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XB6010000 |
| Packaging Group | II; III |
| Hazard Class | 4.1 |
| HS Code | 29012980 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2901299090 |
|---|---|
| Summary | 2901299090 unsaturated acyclic hydrocarbons。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:2.0%。General tariff:30.0% |
|
The mannoprotein TIR3 (CAGL0C03872g) is required for sterol uptake in Candida glabrata.
Biochim. Biophys. Acta 1851(2) , 141-51, (2015) Sterol uptake in the pathogenic fungus, Candida glabrata, occurs via the sterol transporter, CgAus1p. Azole inhibition of sterol biosynthesis can under certain circumstances be reversed by adding exog... |
|
|
Comparison of rheological properties, follicular penetration, drug release, and permeation behavior of a novel topical drug delivery system and a conventional cream.
Eur. J. Pharm. Biopharm. 88(3) , 614-24, (2015) A novel adapalene-loaded solid lipid microparticle (SLMA) dispersion as a topical drug delivery system (TDDS) for follicular penetration has been introduced. The objective of the present study was to ... |
|
|
Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly.
PLoS ONE 9(8) , e105073, (2014) Royal jelly (RJ) intake lowers serum cholesterol levels in animals and humans, but the active component in RJ that lowers serum cholesterol level and its molecular mechanism are unclear. In this study... |
| Squalene, all-trans- |
| (6E,10E,14E,18E)-2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene |
| (E,E,E,E)-Squalene |
| 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene |
| (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene |
| Squalene |
| triacontahexaene |
| Spinacen |
| EINECS 203-826-1 |
| Supraene |
| MFCD00008912 |
| 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (6E,10E,14E,18E)- |
| SQUALENE OIL |
| SPINACENE |
| SQUALENE WITH GC |
| (6E,10E,14E,18E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene |
| Squalen |
| 2,6,10,15,19,23-Hexamethyltetracosa-(2E,6E,10E,14E,18E,22E)-2,6,10,14,18,22-hexaene |
| trans-Spinacene |
![[(6E,10E,14E,18E)-13-(1-ethoxyethoxy)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-12-yl]sulfonylbenzene Structure](https://image.chemsrc.com/caspic/069/1299297-72-5.png) CAS#:1299297-72-5
CAS#:1299297-72-5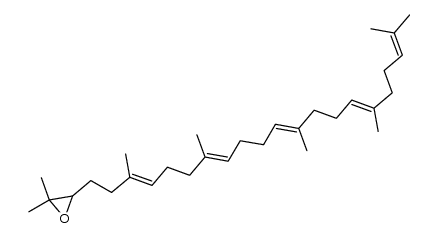 CAS#:14050-42-1
CAS#:14050-42-1 CAS#:24163-93-7
CAS#:24163-93-7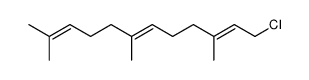 CAS#:6784-45-8
CAS#:6784-45-8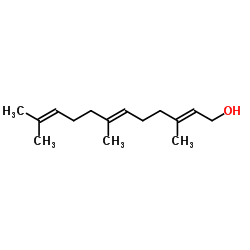 CAS#:106-28-5
CAS#:106-28-5 CAS#:68690-45-9
CAS#:68690-45-9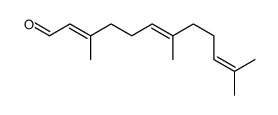 CAS#:502-67-0
CAS#:502-67-0 CAS#:56881-53-9
CAS#:56881-53-9 CAS#:6138-90-5
CAS#:6138-90-5 CAS#:3641-51-8
CAS#:3641-51-8 CAS#:61898-58-6
CAS#:61898-58-6 CAS#:3796-70-1
CAS#:3796-70-1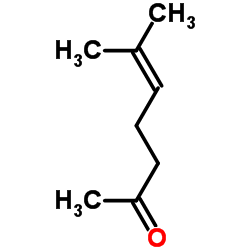 CAS#:110-93-0
CAS#:110-93-0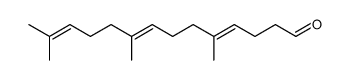 CAS#:67858-78-0
CAS#:67858-78-0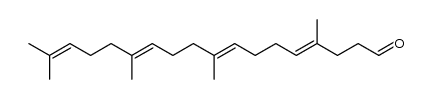 CAS#:56882-09-8
CAS#:56882-09-8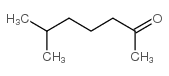 CAS#:928-68-7
CAS#:928-68-7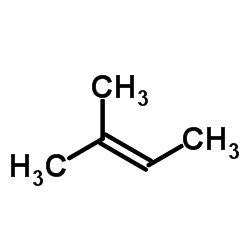 CAS#:513-35-9
CAS#:513-35-9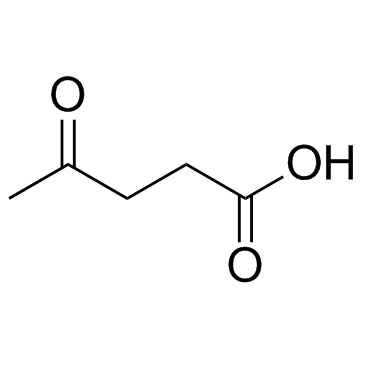 CAS#:123-76-2
CAS#:123-76-2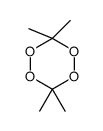 CAS#:1073-91-2
CAS#:1073-91-2
