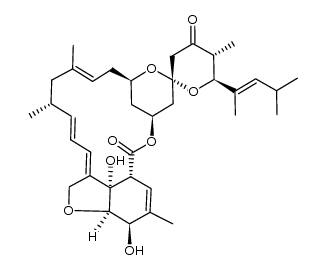
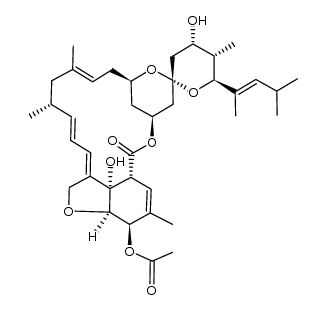
Moxidectin
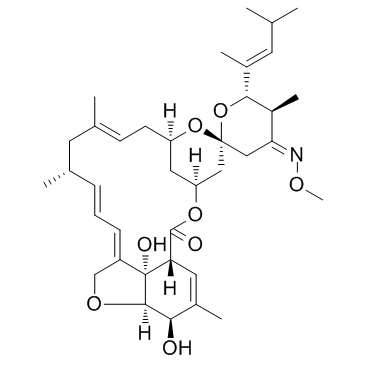
Moxidectin structure
|
Common Name | Moxidectin | ||
|---|---|---|---|---|
| CAS Number | 113507-06-5 | Molecular Weight | 639.819 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 790.0±70.0 °C at 760 mmHg | |
| Molecular Formula | C37H53NO8 | Melting Point | 132 °C | |
| MSDS | Chinese USA | Flash Point | 431.6±35.7 °C | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
Use of MoxidectinMoxidectin(ProHeart 6; CL301423; Cydectin) is an anthelmintic drug which kills parasitic worms (helminths), and is used for the prevention and control of heartworm and intestinal worms.IC50 value:Target: Anti-parasiticMoxidectin is a semisynthetic derivative of nemadectin, which is produced by fermentation by Streptomyces cyano-griseus. Moxidectin treats and controls some of the most common internal and external parasites by selectively binding to a parasite's glutamate-gated chloride ion channels. These channels are vital to the function of invertebrate nerve and muscle cells; when moxidectin binds to the channels, it disrupts neurotransmission, resulting in paralysis and death of the parasite. Studies of moxidectin show the side effects vary by animal and may be affected by the product’s formulation, application method and dosage. |
| Name | moxidectin |
|---|---|
| Synonym | More Synonyms |
| Description | Moxidectin(ProHeart 6; CL301423; Cydectin) is an anthelmintic drug which kills parasitic worms (helminths), and is used for the prevention and control of heartworm and intestinal worms.IC50 value:Target: Anti-parasiticMoxidectin is a semisynthetic derivative of nemadectin, which is produced by fermentation by Streptomyces cyano-griseus. Moxidectin treats and controls some of the most common internal and external parasites by selectively binding to a parasite's glutamate-gated chloride ion channels. These channels are vital to the function of invertebrate nerve and muscle cells; when moxidectin binds to the channels, it disrupts neurotransmission, resulting in paralysis and death of the parasite. Studies of moxidectin show the side effects vary by animal and may be affected by the product’s formulation, application method and dosage. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 790.0±70.0 °C at 760 mmHg |
| Melting Point | 132 °C |
| Molecular Formula | C37H53NO8 |
| Molecular Weight | 639.819 |
| Flash Point | 431.6±35.7 °C |
| Exact Mass | 639.377136 |
| PSA | 116.04000 |
| LogP | 8.43 |
| Vapour Pressure | 0.0±6.2 mmHg at 25°C |
| Index of Refraction | 1.581 |
| InChIKey | YZBLFMPOMVTDJY-XHLLERFLSA-N |
| SMILES | CON=C1CC2(CC3CC(CC=C(C)CC(C)C=CC=C4COC5C(O)C(C)=CC(C(=O)O3)C45O)O2)OC(C(C)=CC(C)C)C1C |
| Storage condition | 2-8°C |
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H400 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T,N |
| Risk Phrases | 25-50 |
| Safety Phrases | 45-61-24/25 |
| RIDADR | UN 2588 |
| RTECS | PY5438800 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29322090 |
|
~81% 
Moxidectin CAS#:113507-06-5 |
| Literature: Tetrahedron Letters, , vol. 29, # 21 p. 2595 - 2598 |
|
~% 
Moxidectin CAS#:113507-06-5 |
| Literature: Tetrahedron Letters, , vol. 29, # 21 p. 2595 - 2598 |
|
~% 
Moxidectin CAS#:113507-06-5 |
| Literature: Tetrahedron Letters, , vol. 29, # 21 p. 2595 - 2598 |
|
Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator".
Int. J. Parasitol. Drugs Drug Resist. 4(3) , 233-43, (2014) A major hindrance to evaluating nematode populations for anthelmintic resistance, as well as for screening existing drugs, new compounds, or bioactive plant extracts for anthelmintic properties, is th... |
|
|
In-vitro activity of avermectins against Mycobacterium ulcerans.
PLoS Negl. Trop. Dis. 9(3) , e0003549, (2015) Mycobacterium ulcerans causes Buruli ulcer (BU), a debilitating infection of subcutaneous tissue. There is a WHO-recommended antibiotic treatment requiring an 8-week course of streptomycin and rifampi... |
|
|
The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer.
EMBO Mol. Med. 6(10) , 1263-78, (2014) Constitutive activation of canonical WNT-TCF signaling is implicated in multiple diseases, including intestine and lung cancers, but there are no WNT-TCF antagonists in clinical use. We have performed... |
| Equest |
| Vetdectin Oral Drench |
| (1'R,2R,4E,4'S,5S,6S,8'R,10'E,13'R,14'E,16'E,20'R,21'R,24'S)-21',24'-Dihydroxy-4-(methoxyimino)-5,11',13',22'-tetramethyl-6-[(2E)-4-methyl-2-penten-2-yl]-3,4,5,6-tetrahydro-2'H-spiro[pyran-2,6'-[3,7,1 ;9]trioxatetracyclo[15.6.1.1.0]pentacosa[10,14,16,22]tetraen]-2'-one |
| proheart |
| bridged fused ring systems nomenclature: (2aE,4E,8E)-(5’S,6R,6’S,11R,13R,15S,17aR,20R,20aR,20bS)-6’-[(1E)-1,3-dimethylbut-1-enyl]-5’,6’,10,11,14,15,17a,20,20a,20b-decahydro-20,20b-dihydroxy-5’,6,8,19-tetramethylspiro[11,15-methano-2H,13H,17H-furo[4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2’-[2H]pyran]-4’,17(3’H,6H)-dione 4’-(E)-(O-methyloxime) |
| ProHeart 6 |
| Moxidectin HOUSE STANDARD |
| Moxidectin |
| (6R,23E,25S)-5-O-demethyl-28-deoxy-25-[(1E)-1,3-dimethyl-1-butenyl]-6,28-epoxy-23-(methoxyimino)milbemycin B |
| Quest |
| extended von Baeyer nomenclature: (10E,14E,16E)-(1R,4S,5’S,6R,6’S,8R,13R,20R,21R,24S)-6’-[(1E)-1,3-dimethylbut-1-enyl]-21,24-dihydroxy-5’,11,13,22-tetramethyl-(3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene)-6-spiro-2’-(tetrahydropyran)-2,4’-dione 4’-(E)-(O-methyloxime) |