Z-Gly-OH

Z-Gly-OH structure
|
Common Name | Z-Gly-OH | ||
|---|---|---|---|---|
| CAS Number | 1138-80-3 | Molecular Weight | 209.199 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 424.0±38.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO4 | Melting Point | 118-122 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 210.2±26.8 °C | |
Use of Z-Gly-OHZ-Glycine is a Glycine (HY-Y0966) derivative[1]. |
| Name | N-benzyloxycarbonylglycine |
|---|---|
| Synonym | More Synonyms |
| Description | Z-Glycine is a Glycine (HY-Y0966) derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 424.0±38.0 °C at 760 mmHg |
| Melting Point | 118-122 °C(lit.) |
| Molecular Formula | C10H11NO4 |
| Molecular Weight | 209.199 |
| Flash Point | 210.2±26.8 °C |
| Exact Mass | 209.068802 |
| PSA | 75.63000 |
| LogP | 1.37 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.554 |
| InChIKey | CJUMAFVKTCBCJK-UHFFFAOYSA-N |
| SMILES | O=C(O)CNC(=O)OCc1ccccc1 |
| Water Solubility | methanol: 0.1 g/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Anticonvulsant activities of N-benzyloxycarbonylglycine after parenteral administration.
Neuroreport 5(7) , 777-80, (1994) Although glycine does not cross easily the blood-brain barrier, it exhibits at very high doses (10-40 mmol kg-1) a modest anticonvulsant activity. In this study, carbamate derivatives--N-benzyloxycarb... |
|
|
Substituted hippurates and hippurate analogs as substrates and inhibitors of peptidylglycine alpha-hydroxylating monooxygenase (PHM).
Bioorg. Med. Chem. 16 , 10061-74, (2008) Peptidyl alpha-hydroxylating monooxygenase (PHM) functions in vivo towards the biosynthesis of alpha-amidated peptide hormones in mammals and insects. PHM is a potential target for the development of ... |
|
|
Synthesis and anticonvulsant activity of N-benzyloxycarbonyl-amino acid prodrugs of phenytoin.
J. Pharm. Pharmacol. 51 , 549-553, (1999) Glycine, which has weak anticonvulsant properties, has been shown to potentiate the activity of several antiepileptic drugs but not phenytoin. Recently, studies have shown that N-(benzyloxycarbonyl)gl... |
| Cbz-Gly-OH |
| CBZ-GLYCINE |
| QV1MVO1R |
| N-[(Benzyloxy)carbonyl]glycine |
| N-Carbobenzoxyglycine |
| Z-GLY-OH |
| CBZ-GLY |
| N-Carbobenzyloxyglycine |
| Z-GLYCINE |
| Glycine, N-[(phenylmethoxy)carbonyl]- |
| z-gly |
| benzyloxycarbonylaminomethylcarboxylic acid |
| EINECS 214-516-0 |
| MFCD00002691 |
| N-Gly-OH |
| N-Cbz-glycine |
| Carbobenzyloxyglycine |
| {[(Benzyloxy)carbonyl]amino}acetic acid |
| Benzyloxycarbonylglycine |
| Benzyloxycarbonyl-glycine |
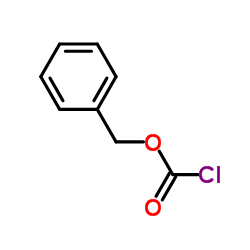 CAS#:501-53-1
CAS#:501-53-1 CAS#:56-40-6
CAS#:56-40-6 CAS#:77987-49-6
CAS#:77987-49-6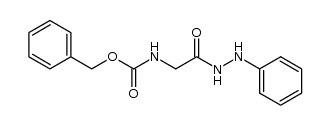 CAS#:21855-71-0
CAS#:21855-71-0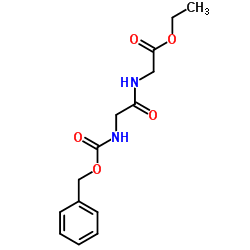 CAS#:1145-81-9
CAS#:1145-81-9 CAS#:16881-32-6
CAS#:16881-32-6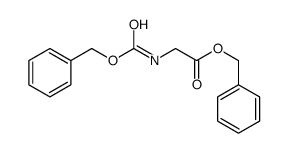 CAS#:5513-38-2
CAS#:5513-38-2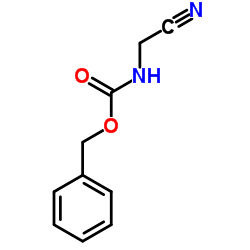 CAS#:3589-41-1
CAS#:3589-41-1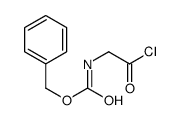 CAS#:15050-24-5
CAS#:15050-24-5![L-Phenylalanine,N-[N-[(phenylmethoxy)carbonyl]glycyl]-, pentachlorophenyl ester (9CI) structure](https://image.chemsrc.com/caspic/178/14131-92-1.png) CAS#:14131-92-1
CAS#:14131-92-1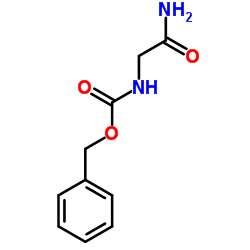 CAS#:949-90-6
CAS#:949-90-6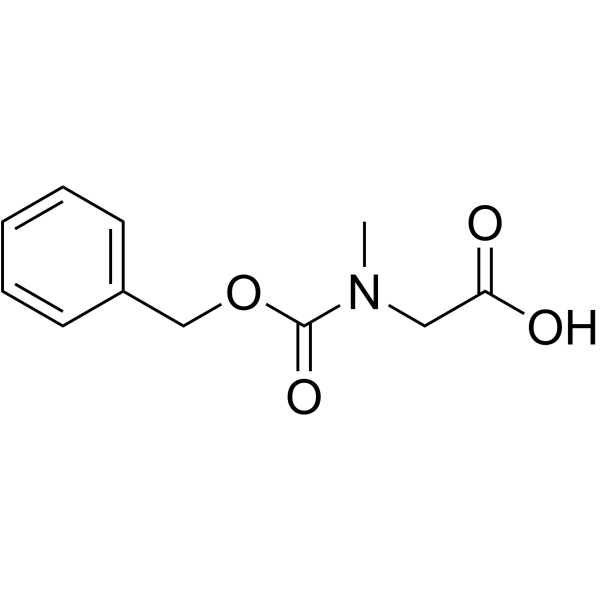 CAS#:39608-31-6
CAS#:39608-31-6 CAS#:1738-68-7
CAS#:1738-68-7![benzyl 2-[(2-phenylmethoxycarbonylaminoacetyl)amino]acetate structure](https://image.chemsrc.com/caspic/220/19525-53-2.png) CAS#:19525-53-2
CAS#:19525-53-2![L-Phenylalanine,N-[N-[(phenylmethoxy)carbonyl]glycyl]-, hydrazide (9CI) structure](https://image.chemsrc.com/caspic/259/17942-42-6.png) CAS#:17942-42-6
CAS#:17942-42-6 CAS#:21855-72-1
CAS#:21855-72-1 CAS#:100-39-0
CAS#:100-39-0
