CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YM4550000
-
CHEMICAL NAME :
-
1-alpha-H,5-alpha-H-Tropan-3-alpha-ol, 6-beta,7-beta-epoxy-, (-)-tropate (ester), hydrobromide
-
CAS REGISTRY NUMBER :
-
114-49-8
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C17-H21-N-O4.Br-H
-
MOLECULAR WEIGHT :
-
384.31
-
WISWESSER LINE NOTATION :
-
T C356 A AN DOTJ A1 HOVYR&1Q &EH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1270 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraduodenal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
670 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1880 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
650 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CLDND* Compilation of LD50 Values of New Drugs. (J.R. MacDougal, Dept. of National Health and Welfare, Food and Drug Divisions, 35 John St., Ottawa, Ont., Canada)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1650 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
203 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
80 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - other changes Vascular - BP lowering not characterized in autonomic section Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - general anesthetic Behavioral - convulsions or effect on seizure threshold
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
850 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - parasympatholytic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
975 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
975 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2365 mg/kg
-
SEX/DURATION :
-
female 10-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Human HeLa cell
-
DOSE/DURATION :
-
1 pph/5H
-
REFERENCE :
-
HUMAA7 Humangenetik. (Heidelberg, Fed. Rep. Ger.) V.1-30, 1964-75. For publisher information, see HUGEDQ. Volume(issue)/page/year: 4,371,1967 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80511 No. of Facilities: 1011 (estimated) No. of Industries: 3 No. of Occupations: 11 No. of Employees: 7730 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80511 No. of Facilities: 462 (estimated) No. of Industries: 1 No. of Occupations: 5 No. of Employees: 19132 (estimated) No. of Female Employees: 16485 (estimated)
|
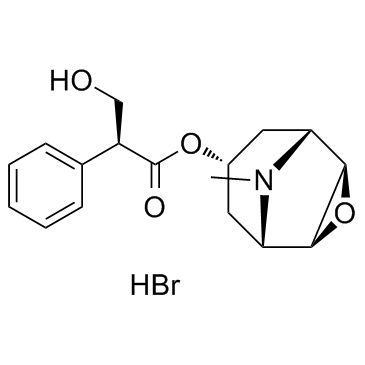
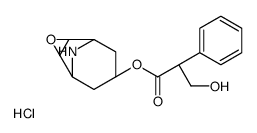 CAS#:24676-80-0
CAS#:24676-80-0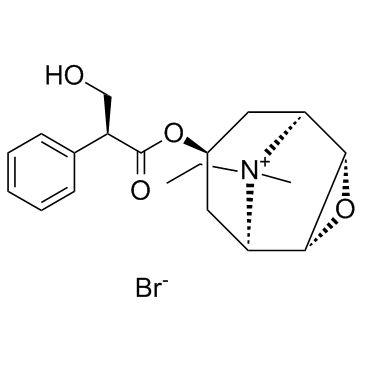 CAS#:30286-75-0
CAS#:30286-75-0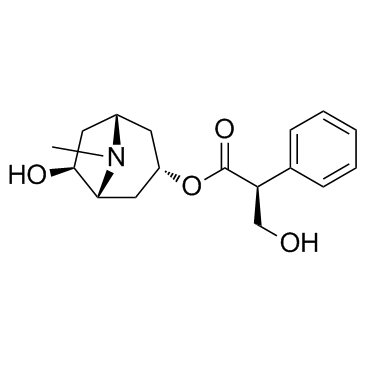 CAS#:55869-99-3
CAS#:55869-99-3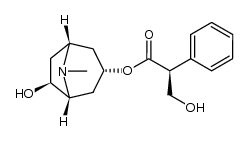 CAS#:126371-43-5
CAS#:126371-43-5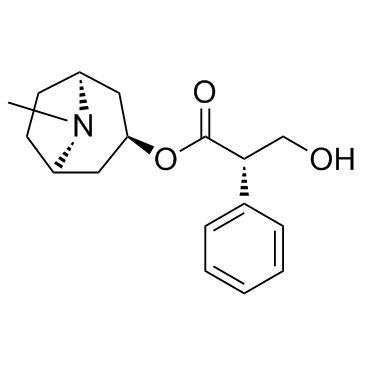 CAS#:101-31-5
CAS#:101-31-5![3-Oxa-9-azatricyclo[3.3.1.02,4]non-7-yl tropate structure](https://www.chemsrc.com/caspic/204/4684-28-0.png) CAS#:4684-28-0
CAS#:4684-28-0