Avagacestat
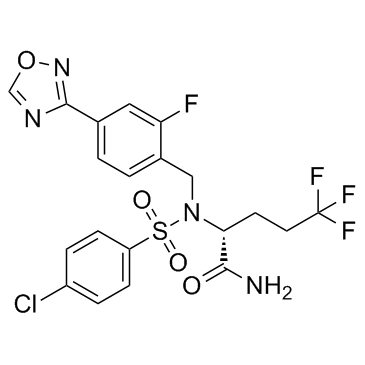
Avagacestat structure
|
Common Name | Avagacestat | ||
|---|---|---|---|---|
| CAS Number | 1146699-66-2 | Molecular Weight | 520.885 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 652.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C20H17ClF4N4O4S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 348.3±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AvagacestatBMS-708163 is a potent inhibitor of γ-secretase, with IC50s of 0.27 nM and 0.30 nM for Aβ42 and Aβ40 inhibition; BMS-708163 also inhibits NICD (Notch IntraCellular Domain) with IC50 of 0.84 nM and shows weak inhibition of CYP2C19, with IC50 of 20 μM. |
| Name | (2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide |
|---|---|
| Synonym | More Synonyms |
| Description | BMS-708163 is a potent inhibitor of γ-secretase, with IC50s of 0.27 nM and 0.30 nM for Aβ42 and Aβ40 inhibition; BMS-708163 also inhibits NICD (Notch IntraCellular Domain) with IC50 of 0.84 nM and shows weak inhibition of CYP2C19, with IC50 of 20 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.27 nM (γ-secretase, Aβ42), 0.30 nM (γ-secretase, Aβ40), 20 μM (CYP2C19)[1], 0.84 nM (NICD)[2] |
| In Vitro | BMS-708163 exhibits weaker potency for inhibition of Notch processing, IC50=58±23 nM, as compared to its inhibition potency for APP cleavage[1]. BMS-708163 (10 µM) combined with gefitinib significantly attenuates the colony growth of PC9/AB2 cells, increases the expression of active caspase 3 and PARP and reduces the expression of Ki-67 in PC9/AB2 cells. BMS-708163 induces apoptosis and enhances cell cycle arrest at the G1 phase in PC9/AB2 cells. BMS-708163 treatment effectively downregulates the expression of Notch1, HES1, PI3K and Akt in PC9/AB2 cells[3]. |
| In Vivo | BMS-708163 significantly reduces both plasma and brain Aβ40 levels relative to control at 10 and 100 mg/kg for the entire dosing interval, demonstrates significant Aβ40 lowering for 8 h after an oral dose of 1 mg/kg, and significantly lowers CSF Aβ40 levels in rats, when measured 5 h after single oral doses ranging from 3 to 100 mg/kg[1]. BMS-708163 (10 mg/kg) monotherapy has a minor inhibitory effect on PC9/AB2 tumor growth compared with gefitinib alone. BMS-708163 monotherapy results in a slight increase in caspase 3 expression as well as a mild decrease in Ki-67 expression in vivo. In the xenograft lung cancer samples treated with BMS-708163 plus gefitinib, there are a marked increase in caspase 3 expression and a reduction in Ki-67 staining[3]. |
| Cell Assay | The cell viability is assessed using a tetrazolium salt (WST-8)-based colorimetric assay from the Cell Counting Kit 8 (CCK-8). The cells are seeded into 96-well plates at an initial density of 5×103 cells/well and cultured for 24 h, after which the cells are cultured with DMSO, increased concentrations of gefitinib or BMS708163, BIBW2992, or the combination of BMS708163 and BIBW2992 for an additional 48 h. The A450 is measured in a microplate reader after 10 µL of CCK-8 solution is added and incubated for 1 h. The percentage of growth is shown relative to untreated controls. |
| Animal Admin | Four- to six-week-old female Balb/c athymic (nu + /nu +) mice are anesthetized with ether. The mice are acclimatized for one week before being injected with 1.5×106 PC9/AB2 cells that have been resuspended in 200 μL of matrigel. When established tumors of approximately 150-300 mm3 in diameter are detected, the mice are randomly divided into groups and fed orally by gavage with either vehicle (1% methylcellulose, 0.2% Tween 80 in sterilized water), gefitinib (3 mg/kg diluted in vehicle), BMS-708163 (10 mg/kg diluted in vehicle), or a combination of gefitinib (3 mg/kg) and BMS-708163 (10 mg/kg) for 5 days/week. Each treatment group consists of eight mice. The tumor volume are measured and calculated every five days using the following formula: π/6×(larger diameter)×(smaller diameter)2. After 30 days, mice are killed by cervical dislocation. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 652.3±65.0 °C at 760 mmHg |
| Molecular Formula | C20H17ClF4N4O4S |
| Molecular Weight | 520.885 |
| Flash Point | 348.3±34.3 °C |
| Exact Mass | 520.059509 |
| PSA | 127.77000 |
| LogP | 4.89 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.564 |
| InChIKey | XEAOPVUAMONVLA-QGZVFWFLSA-N |
| SMILES | NC(=O)C(CCC(F)(F)F)N(Cc1ccc(-c2ncon2)cc1F)S(=O)(=O)c1ccc(Cl)cc1 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
|
~90% 
Avagacestat CAS#:1146699-66-2 |
| Literature: Bristol-Myers Squibb Company Patent: US2009/111858 A1, 2009 ; Location in patent: Page/Page column 23 ; |
|
~49% 
Avagacestat CAS#:1146699-66-2 |
| Literature: Bristol-Myers Squibb Company Patent: US2009/111858 A1, 2009 ; Location in patent: Page/Page column 21-22 ; |
|
~43% 
Avagacestat CAS#:1146699-66-2 |
| Literature: Bristol-Myers Squibb Company Patent: US2009/111858 A1, 2009 ; Location in patent: Page/Page column 4; 19 ; |
|
~% 
Avagacestat CAS#:1146699-66-2 |
| Literature: US2009/111858 A1, ; Page/Page column 20-21 ; |
| (R)-2-(4-chloro-N-(2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl)phenylsulfonamido)-5,5,5-trifluoropentanamide |
| BMS-708163 |
| Avagacestat |
| Pentanamide, 2-[[(4-chlorophenyl)sulfonyl][[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoro-, (2R)- |
| N-[(4-Chlorophenyl)sulfonyl]-5,5,5-trifluoro-N-[2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl]norvalinamide |

![Pentanamide, 2-[[(4-chlorophenyl)sulfonyl]amino]-5,5,5-trifluoro-, (2R)- structure](https://image.chemsrc.com/caspic/312/1146699-67-3.png)
