Trabectedin
Modify Date: 2024-01-02 18:40:30

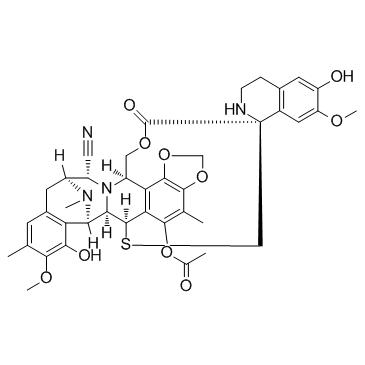
Trabectedin structure
|
Common Name | Trabectedin | ||
|---|---|---|---|---|
| CAS Number | 114899-77-3 | Molecular Weight | 761.837 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C39H43N3O11S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TrabectedinTrabectedin (Ecteinascidin-743 or ET-743) is a novel antitumour agent of marine origin with potent antitumour activity both in vitro and in vivo.IC50 Value: 0.1-3.7 nM (breast cancer cell lines) [1]Target: Apoptosis inducer; Anticancerin vitro: Trabectedin induced cytotoxicity and apoptosis in both breast cancer cells in a time and concentration-dependent manner. The expression levels of the death receptor pathway molecules, TRAIL-R1/DR4, TRAIL-R2/DR5, FAS/TNFRSF6, TNF RI/TNFRSF1A, and FADD were significantly increased by 2.6-, 3.1-, 1.7-, 11.2- and 4.0-fold by trabectedin treatment in MCF-7 cells. However, in MDA-MB-453 cells, the mitochondrial pathway related pro-apoptotic proteins Bax, Bad, Cytochrome c, Smac/DIABLO, and Cleaved Caspase-3 expressions were induced by 4.2-, 3.6-, 4.8-, 4.5-, and 4.4-fold, and the expression levels of anti-apoptotic proteins Bcl-2 and Bcl-XL were reduced by 4.8- and 5.2-fold in MDA-MB-453 cells [2]. In vitro treatment with noncytotoxic concentrations of trabectedin selectively inhibited the production of CCL2, CXCL8, IL-6, VEGF, and PTX3 by MLS primary tumor cultures and/or cell lines [3].in vivo: A xenograft mouse model of human MLS showed marked reduction of CCL2, CXCL8, CD68+ infiltrating macrophages, CD31+ tumor vessels, and partial decrease of PTX3 after trabectedin treatment [3]. The MTD of trabectedin was 700 microg/m(2) due to dose-limiting neutropaenia and the RDs in the previously treated/untreated patients were 500 and 600 microg/m(2), respectively. Most common toxicities were nausea/vomiting (67%), asthenia/fatigue (55%) and reversible ASAT/ALAT elevation (51%) [4]. Toxicity: Most common toxicities were nausea/vomiting (67%), asthenia/fatigue (55%) and reversible ASAT/ALAT elevation (51%) [4].Clinical trial: A Study to Assess the Potential Effects of Rifampin on the Pharmacokinetics of Trabectedin in Patients With Advanced Malignancies. Phase 1/2 |
| Name | trabectedin |
|---|---|
| Synonym | More Synonyms |
| Description | Trabectedin (Ecteinascidin-743 or ET-743) is a novel antitumour agent of marine origin with potent antitumour activity both in vitro and in vivo.IC50 Value: 0.1-3.7 nM (breast cancer cell lines) [1]Target: Apoptosis inducer; Anticancerin vitro: Trabectedin induced cytotoxicity and apoptosis in both breast cancer cells in a time and concentration-dependent manner. The expression levels of the death receptor pathway molecules, TRAIL-R1/DR4, TRAIL-R2/DR5, FAS/TNFRSF6, TNF RI/TNFRSF1A, and FADD were significantly increased by 2.6-, 3.1-, 1.7-, 11.2- and 4.0-fold by trabectedin treatment in MCF-7 cells. However, in MDA-MB-453 cells, the mitochondrial pathway related pro-apoptotic proteins Bax, Bad, Cytochrome c, Smac/DIABLO, and Cleaved Caspase-3 expressions were induced by 4.2-, 3.6-, 4.8-, 4.5-, and 4.4-fold, and the expression levels of anti-apoptotic proteins Bcl-2 and Bcl-XL were reduced by 4.8- and 5.2-fold in MDA-MB-453 cells [2]. In vitro treatment with noncytotoxic concentrations of trabectedin selectively inhibited the production of CCL2, CXCL8, IL-6, VEGF, and PTX3 by MLS primary tumor cultures and/or cell lines [3].in vivo: A xenograft mouse model of human MLS showed marked reduction of CCL2, CXCL8, CD68+ infiltrating macrophages, CD31+ tumor vessels, and partial decrease of PTX3 after trabectedin treatment [3]. The MTD of trabectedin was 700 microg/m(2) due to dose-limiting neutropaenia and the RDs in the previously treated/untreated patients were 500 and 600 microg/m(2), respectively. Most common toxicities were nausea/vomiting (67%), asthenia/fatigue (55%) and reversible ASAT/ALAT elevation (51%) [4]. Toxicity: Most common toxicities were nausea/vomiting (67%), asthenia/fatigue (55%) and reversible ASAT/ALAT elevation (51%) [4].Clinical trial: A Study to Assess the Potential Effects of Rifampin on the Pharmacokinetics of Trabectedin in Patients With Advanced Malignancies. Phase 1/2 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C39H43N3O11S |
| Molecular Weight | 761.837 |
| Exact Mass | 761.261841 |
| PSA | 194.02000 |
| LogP | 3.10 |
| Index of Refraction | 1.732 |
| Storage condition | -20°C |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| ecteinascidin-743 |
| (1R,1'R,2'R,3'R,11'S,12'S,14'R)-5',6,12'-Trihydroxy-6',7-dimethoxy-7',21',30'-trimethyl-27'-oxo-3,4-dihydro-2H-spiro[isoquinoline-1,26'-[17,19,28]trioxa[24]thia[13,30]diazaheptacyclo[12.9.6.1.0.0.0.0]triaconta[4,6,8,15,20,22]hexaen]-22'-yl acetate |
| ET-743 |
| Trabectedin |
| ecteinascidin 743 |
| (1'R,6R,6aR,7R,13S,14S,16R) |
| Ecteinascidine 743 |
| Ecteinascidin |
| ET743 |
| Yondelis(R) |
| UNII-ID0YZQ2TCP |
| 5-(acetyloxy)-3',4',6,6a,7,13,14,16-octahydro-6',8,14-trihydroxy-7',9-dimethoxy-4,10,23-trimethyl-,[6R-(6a,6a3,73,133,143,16a,20R*)] |
| Yondelis |
 CAS#:114899-80-8
CAS#:114899-80-8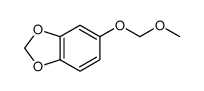 CAS#:111726-43-3
CAS#:111726-43-3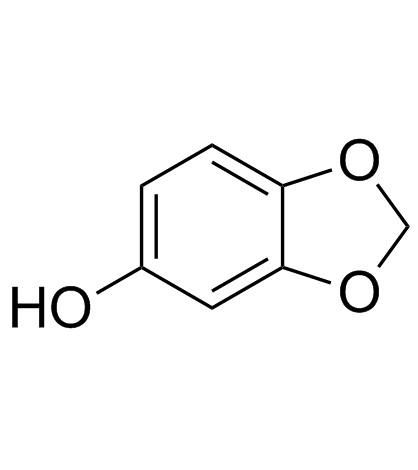 CAS#:533-31-3
CAS#:533-31-3 CAS#:244126-41-8
CAS#:244126-41-8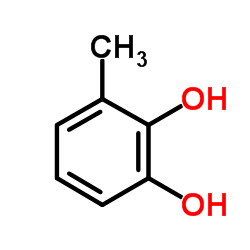 CAS#:488-17-5
CAS#:488-17-5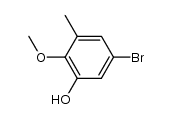 CAS#:244229-56-9
CAS#:244229-56-9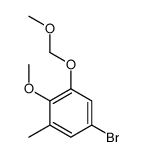 CAS#:244126-25-8
CAS#:244126-25-8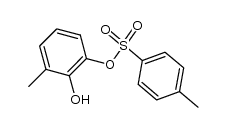 CAS#:244126-22-5
CAS#:244126-22-5 CAS#:244126-24-7
CAS#:244126-24-7