2-Carboxybenzaldehyde
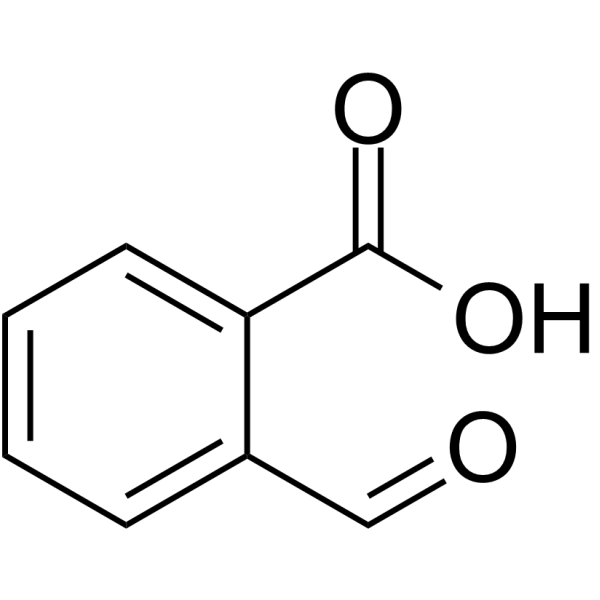
2-Carboxybenzaldehyde structure
|
Common Name | 2-Carboxybenzaldehyde | ||
|---|---|---|---|---|
| CAS Number | 119-67-5 | Molecular Weight | 150.13 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 321.8±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H6O3 | Melting Point | 94-96 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 162.6±19.7 °C | |
Use of 2-Carboxybenzaldehyde2-Carboxybenzaldehyde is a chemical compound. 2-Carboxybenzaldehyde consists of a benzene ring, with an aldehyde and a carboxylic acid as substituents that are ortho to each other. 2-Carboxybenzaldehyde can be used to form ligands with metal ions. Ni(II) and Cu(II) complexes of 2-Carboxybenzaldehyde thiosemicarbazone has anticancer and antibacterial activities[1]. |
| Name | 2-formylbenzoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Carboxybenzaldehyde is a chemical compound. 2-Carboxybenzaldehyde consists of a benzene ring, with an aldehyde and a carboxylic acid as substituents that are ortho to each other. 2-Carboxybenzaldehyde can be used to form ligands with metal ions. Ni(II) and Cu(II) complexes of 2-Carboxybenzaldehyde thiosemicarbazone has anticancer and antibacterial activities[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 321.8±25.0 °C at 760 mmHg |
| Melting Point | 94-96 °C(lit.) |
| Molecular Formula | C8H6O3 |
| Molecular Weight | 150.13 |
| Flash Point | 162.6±19.7 °C |
| Exact Mass | 150.031693 |
| PSA | 54.37000 |
| LogP | 0.77 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.620 |
| InChIKey | DYNFCHNNOHNJFG-UHFFFAOYSA-N |
| SMILES | O=Cc1ccccc1C(=O)O |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | PG2 |
| WGK Germany | 2 |
| RTECS | TH7015000 |
| HS Code | 29183000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Highly efficient synthesis of N-substituted isoindolinones and phthalazinones using Pt nanowires as catalysts.
Org. Lett. 14(7) , 1876-9, (2012) A series of N-substituted isoindolinones have been successfully synthesized through the reductive C-N coupling and intramolecular amidation of 2-carboxybenzaldehyde and amines. This one-pot synthesis ... |
|
|
Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR-1:isolation and identification of metabolites.
J. Appl. Microbiol. 85(4) , 746-54, (1998) The findings from a biodegradability study of fluoranthene using two pure bacterial strains, Pasteurella sp. IFA (B-2) and Mycobacterium sp. PYR-1 (AM) are reported. Of total fluoranthene, 24% (B-2) a... |
|
|
Degradation of phenanthrene by the rhizobacterium Ensifer meliloti.
Biodegradation 25(6) , 787-95, (2014) The biodegradation of the polycyclic aromatic hydrocarbon phenantherene by the rhizobacterial strain Ensifer meliloti P221, isolated from the root zone of plant grown in PAH-contaminated soil was stud... |
| Phthalaldehydic Acid |
| Benzoic acid, 2-formyl- |
| EINECS 204-342-3 |
| Benzaldehyde-2-carboxylic Acid |
| 2-carboxybenzaldehyde |
| 2-Formylbenzoic acid |
| MFCD00003336 |
| 2-Formyl-benzoic acid |
 CAS#:95-47-6
CAS#:95-47-6 CAS#:496-14-0
CAS#:496-14-0 CAS#:89-98-5
CAS#:89-98-5 CAS#:528-46-1
CAS#:528-46-1 CAS#:6940-49-4
CAS#:6940-49-4 CAS#:29337-71-1
CAS#:29337-71-1![bicyclo[4.2.0]octa-1,3,5-triene-7,8-dione Structure](https://image.chemsrc.com/caspic/201/6383-11-5.png) CAS#:6383-11-5
CAS#:6383-11-5 CAS#:110-89-4
CAS#:110-89-4 CAS#:643-79-8
CAS#:643-79-8 CAS#:529-19-1
CAS#:529-19-1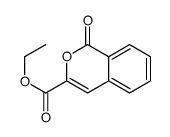 CAS#:111686-63-6
CAS#:111686-63-6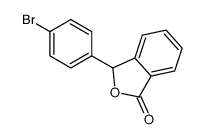 CAS#:25933-36-2
CAS#:25933-36-2 CAS#:112165-32-9
CAS#:112165-32-9 CAS#:107517-71-5
CAS#:107517-71-5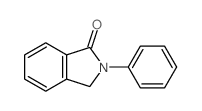 CAS#:5388-42-1
CAS#:5388-42-1 CAS#:4743-58-2
CAS#:4743-58-2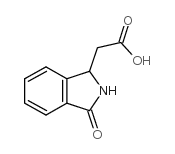 CAS#:3849-22-7
CAS#:3849-22-7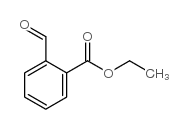 CAS#:34046-43-0
CAS#:34046-43-0 CAS#:3598-68-3
CAS#:3598-68-3![6-phenyl-6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione structure](https://image.chemsrc.com/caspic/177/383148-94-5.png) CAS#:383148-94-5
CAS#:383148-94-5
