UR-1102
Modify Date: 2024-01-14 12:50:55
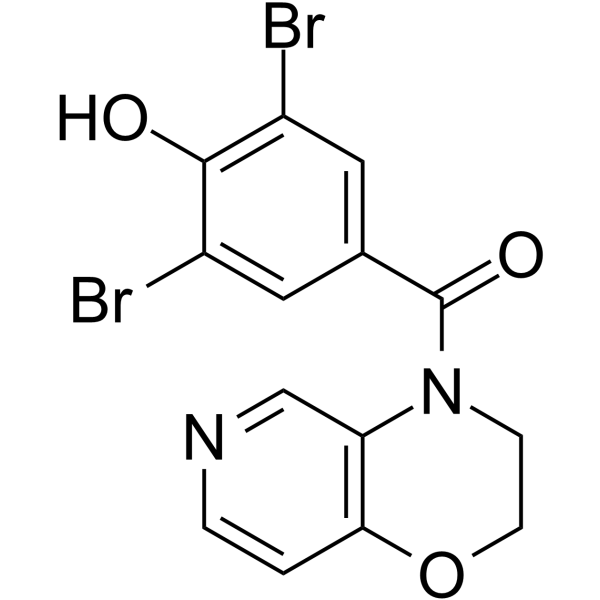
UR-1102 structure
|
Common Name | UR-1102 | ||
|---|---|---|---|---|
| CAS Number | 1198153-15-9 | Molecular Weight | 414.049 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 530.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C14H10Br2N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 274.3±30.1 °C | |
Use of UR-1102Epaminurad (UR-1102) is an orally active, potent and selective URAT1 (urate transporter 1) inhibitor, with a Ki of 0.057 μM. Epaminurad quite modestly inhibits OAT1 and OAT3 (organic anion transporter). Epaminurad is a uricosuric agent. Epaminurad can be used for gout and hyperuricemia research[1]. |
| Name | (3,5-Dibromo-4-hydroxyphenyl)(2,3-dihydro-4H-pyrido[4,3-b][1,4]oxazin-4-yl)methanone |
|---|---|
| Synonym | More Synonyms |
| Description | Epaminurad (UR-1102) is an orally active, potent and selective URAT1 (urate transporter 1) inhibitor, with a Ki of 0.057 μM. Epaminurad quite modestly inhibits OAT1 and OAT3 (organic anion transporter). Epaminurad is a uricosuric agent. Epaminurad can be used for gout and hyperuricemia research[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.057 ± 0.036 μM (URAT1), 2.4 ± 0.2 μM (OAT3), 7.2 ± 0.8 μM (OAT1)[1]. |
| In Vitro | UR-1102 (0-12 μM) inhibits urate and PAH (p-aminohippuric acid) uptake by HEK293 cells transiently expressing URAT1, OAT1, or OAT3[1]. |
| In Vivo | Epaminurad (0-30 mg/kg, Orally, once a day for 3 consecutive days) shows uricosuric and urate-lowering effects[1]. Epaminurad (3-30 mg/kg, Orally, once) shows a good pharmacokinetic profile, increases the fractional excretion of urinary uric acid, and reduces plasma uric acid more effectively[1]. Pharmacokinetic Parameters of Epaminurad (UR-1102) in tufted capuchin monkeys[1]. Group 3 mg/kg 10 mg/kg 30 mg/kg Cmax (μg/mL) 8.96 ± 1.74 42.4 ± 12.8 92.9 ± 21.0 Tmax (h) 0.6 ± 0.2 0.5 ± 0.0 0.8 ± 0.3 T1/2 (h) 4.7 ± 0.9 4.2 ± 1.1 3.3 ± 0.8 AUC0-inf (mg*h/mL) 26.2 ± 8.1 108 ± 51 257 ± 60 Animal Model: Tufted capuchin monkeys[1] Dosage: 0, 3, 10, and 30 mg/kg Administration: Orally, once a day, for 3 consecutive days Result: Showed good uricosuric and urate-lowering effects at 3 mg/kg, the lowest dose, which were comparable to those of benzbromarone at 100 mg/kg, the highest dose, with maximum efficacy. Animal Model: Tufted capuchin monkeys[1] Dosage: 0, 3, 10, and 30 mg/kg Administration: Orally, once Result: Showed a good pharmacokinetic profile. Exhibited both good systemic exposure and significantly great plasma urate-lowering at 3 mg/kg. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 530.0±50.0 °C at 760 mmHg |
| Molecular Formula | C14H10Br2N2O3 |
| Molecular Weight | 414.049 |
| Flash Point | 274.3±30.1 °C |
| Exact Mass | 411.905792 |
| LogP | 3.05 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.691 |
| (3,5-Dibromo-4-hydroxyphenyl)(2,3-dihydro-4H-pyrido[4,3-b][1,4]oxazin-4-yl)methanone |
| Methanone, (3,5-dibromo-4-hydroxyphenyl)(2,3-dihydro-4H-pyrido[4,3-b]-1,4-oxazin-4-yl)- |