GPP78
Modify Date: 2025-09-18 12:15:14
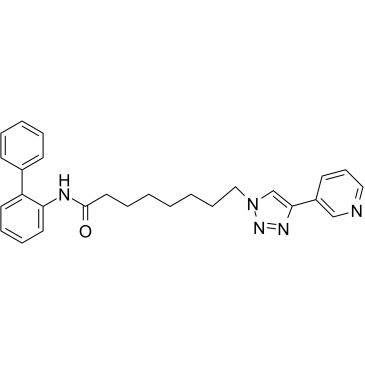
GPP78 structure
|
Common Name | GPP78 | ||
|---|---|---|---|---|
| CAS Number | 1202580-59-3 | Molecular Weight | 439.552 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C27H29N5O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of GPP78GPP78 (CAY10618) is a potent Nampt inhibitor with an IC50 of 3.0 nM for nicotinamide adenine dinucleotide (NAD) depletion. GPP78 is cytotoxic to neuroblastoma cell line SH-SY5Y cells with an IC50 of 3.8 nM by inducing autophagy. GPP78 has anti-cancer and anti-inflammatory effects[1][2]. |
| Name | N-(2-phenylphenyl)-8-(4-pyridin-3-yltriazol-1-yl)octanamide |
|---|---|
| Synonym | More Synonyms |
| Description | GPP78 (CAY10618) is a potent Nampt inhibitor with an IC50 of 3.0 nM for nicotinamide adenine dinucleotide (NAD) depletion. GPP78 is cytotoxic to neuroblastoma cell line SH-SY5Y cells with an IC50 of 3.8 nM by inducing autophagy. GPP78 has anti-cancer and anti-inflammatory effects[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Nampt[1]; Autophagy[1] |
| In Vitro | GPP78 (Compound 8; 10 nM; 24-40 hours; SH-SY5Y cells) treatment with cells, punctate staining of LC3-II and the formation of autophagolysosomes are observable. LC3-II is membrane-bound and is present in autophagosomes[1]. GPP78 (Compound 8) inhibits the growth of most cell lines tested, with nanomolar potency (GI50) in cell lines derived from leukemia, lung, CNS, colon, melanoma, ovarian, renal, and prostate cancers. GPP78 appears truly cytotoxic in melanoma cell lines, while in the others it is mainly cytostatic[1]. Western Blot Analysis[1] Cell Line: SH-SY5Y cells Concentration: 10 nM Incubation Time: 24 hours, 40 hours Result: Punctate staining of LC3-II and the formation of autophagolysosomes were observable. |
| In Vivo | GPP78 (10 mg/kg; intraperitoneal injection; daily; 1 hour or 6 hours after SCI; for 19 days; male adult CD1 mice) treatment reduces the severity of spinal cord trauma in SCI mice[2]. Animal Model: Male adult CD1 mice (25-30 g) with spinal cord injury (SCI)[2] Dosage: 10 mg/kg Administration: Intraperitoneal injection; daily; 1 hour or 6 hours after SCI; for 19 days Result: Significantly reduced the demyelination associated with SCI. And significantly ameliorated the functional deficits induced by SCI. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C27H29N5O |
| Molecular Weight | 439.552 |
| Exact Mass | 439.237213 |
| PSA | 72.70000 |
| LogP | 3.97 |
| Index of Refraction | 1.623 |
| Storage condition | -20°C |
|
~78% 
GPP78 CAS#:1202580-59-3 |
| Literature: Colombano, Giampiero; Travelli, Cristina; Galli, Ubaldina; Caldarelli, Antonio; Chini, Maria Giovanna; Canonico, Pier Luigi; Sorba, Giovanni; Bifulco, Giuseppe; Tron, Gian Cesare; Genazzani, Armando A. Journal of Medicinal Chemistry, 2010 , vol. 53, # 2 p. 616 - 623 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| 1H-1,2,3-Triazole-1-octanamide, N-[1,1'-biphenyl]-2-yl-4-(3-pyridinyl)- |
| N-(2-Biphenylyl)-8-[4-(3-pyridinyl)-1H-1,2,3-triazol-1-yl]octanamide |
![8-[4-(3-pyridyl)-1H-1,2,3-triazol-1-yl]octanoic acid structure](https://image.chemsrc.com/caspic/399/1202580-56-0.png)