3-Indoleacrylic acid

3-Indoleacrylic acid structure
|
Common Name | 3-Indoleacrylic acid | ||
|---|---|---|---|---|
| CAS Number | 1204-06-4 | Molecular Weight | 187.195 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 432.8±20.0 °C at 760 mmHg | |
| Molecular Formula | C11H9NO2 | Melting Point | 180-186ºC | |
| MSDS | USA | Flash Point | 215.6±21.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3-Indoleacrylic acid3-Indoleacrylic acid is a high-efficient antialgal agent. 3-Indoleacrylic acid increases reactive oxygen species (ROS) production, and inhibits the functions of all the nutrient assimilating genes, down-regulated ribulose-1,5-bisphosphate carboxylase/oxygenase II, and cytochrome f genes in P. donghaiense[1]. |
| Name | 3-Indoleacrylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Indoleacrylic acid is a high-efficient antialgal agent. 3-Indoleacrylic acid increases reactive oxygen species (ROS) production, and inhibits the functions of all the nutrient assimilating genes, down-regulated ribulose-1,5-bisphosphate carboxylase/oxygenase II, and cytochrome f genes in P. donghaiense[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 432.8±20.0 °C at 760 mmHg |
| Melting Point | 180-186ºC |
| Molecular Formula | C11H9NO2 |
| Molecular Weight | 187.195 |
| Flash Point | 215.6±21.8 °C |
| Exact Mass | 187.063324 |
| PSA | 53.09000 |
| LogP | 2.34 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.750 |
| InChIKey | PLVPPLCLBIEYEA-UHFFFAOYSA-N |
| SMILES | O=C(O)C=Cc1c[nH]c2ccccc12 |
| Storage condition | 0-6°C |
| Water Solubility | freely soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NL3680000 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators.
J. Med. Chem. 54 , 5320, (2011) Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance. IDO inhibition is thus an active... |
|
|
Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K.
J. Med. Chem. 52 , 2786-93, (2009) Human kynurenine aminotransferase I (hKAT I) catalyzes the formation of kynurenic acid, a neuroactive compound. Here, we report three high-resolution crystal structures (1.50-1.55 A) of hKAT I that ar... |
|
|
NMR studies of the mode of binding of corepressors and inducers to Escherichia coli trp repressor.
Eur. J. Biochem. 235(3) , 804-13, (1996) The binding of the corepressors tryptophan and 5-methyltryptophan and of the inducers 3-indolepropionate, 3-indoleacrylate and 5-methylindole to the Escherichia coli trp repressor have been studied by... |
| 3-(3-Indolyl)acrylic acid,IAA |
| 2-Propenoic acid, 3-(1H-indol-2-yl)-, (2E)- |
| 3-(3-Indolyl)acrylic acid IAA |
| (2E)-3-(1H-Indol-2-yl)acrylic acid |
| 2-Propenoic acid,3-(1H-indol-3-yl)- |
| 3-Indolylacrylic acid |
| 3-Indoleacrylic Acid |
| 3-(3-Indolyl)acrylic Acid |
| EINECS 214-872-7 |
| MFCD00005633 |
| (E)-3-(indol-2-yl)acrylic acid |
| 3-(1H-Indol-3-yl)acrylic acid |
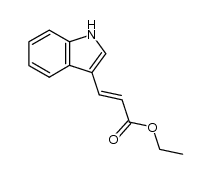 CAS#:110110-37-7
CAS#:110110-37-7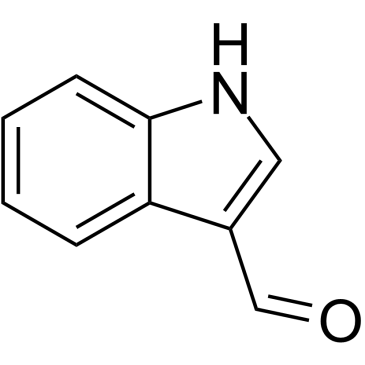 CAS#:487-89-8
CAS#:487-89-8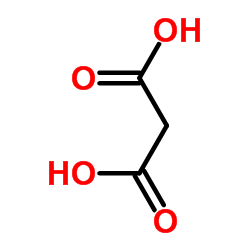 CAS#:141-82-2
CAS#:141-82-2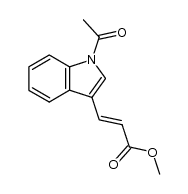 CAS#:19626-93-8
CAS#:19626-93-8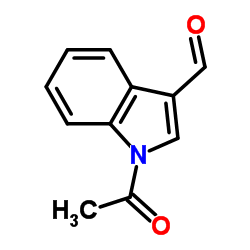 CAS#:22948-94-3
CAS#:22948-94-3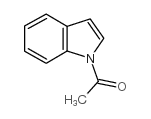 CAS#:576-15-8
CAS#:576-15-8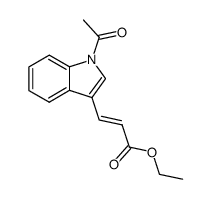 CAS#:87894-44-8
CAS#:87894-44-8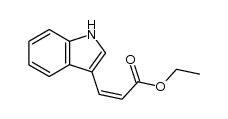 CAS#:110110-44-6
CAS#:110110-44-6 CAS#:830-96-6
CAS#:830-96-6
