UNC0321
Modify Date: 2025-08-23 10:51:04
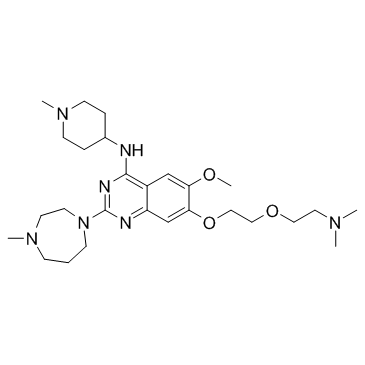
UNC0321 structure
|
Common Name | UNC0321 | ||
|---|---|---|---|---|
| CAS Number | 1238673-32-9 | Molecular Weight | 515.691 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 664.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C27H45N7O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 355.8±34.3 °C | |
Use of UNC0321UNC0321 is a potent and selective G9a inhibitor with Ki of 63 pM, UNC0321 is the first G9a inhibitor with picomolar potency and the most potent G9a inhibitor to date.IC50 value: 63 pM(Ki); 9 nM (ECSD assay) [1]Target: G9aIt was found that replacing the 5-carbon chain in compound 13 with an ethoxyethyl chain resulted in compound 29{UNC0321} (IC50 = 6 nM (CLOT) and 9 nM (ECSD)), the most potent G9a inhibitor to date. 29 had a Morrison Ki of 63 pM and was about 40-fold more potent than 10 (Morrison Ki = 2.6 nM) and 250-fold more potent than 3a (Morrison Ki = 16 nM) in this G9a assay. UNC0321 potentially useful small molecule tools for the biomedical research community to investigate the biology of G9a and its role in chromatin remodeling as well as PTMs of other proteins. |
| Name | 7-{2-[2-(Dimethylamino)ethoxy]ethoxy}-6-methoxy-2-(4-methyl-1,4-d iazepan-1-yl)-N-(1-methyl-4-piperidinyl)-4-quinazolinamine |
|---|---|
| Synonym | More Synonyms |
| Description | UNC0321 is a potent and selective G9a inhibitor with Ki of 63 pM, UNC0321 is the first G9a inhibitor with picomolar potency and the most potent G9a inhibitor to date.IC50 value: 63 pM(Ki); 9 nM (ECSD assay) [1]Target: G9aIt was found that replacing the 5-carbon chain in compound 13 with an ethoxyethyl chain resulted in compound 29{UNC0321} (IC50 = 6 nM (CLOT) and 9 nM (ECSD)), the most potent G9a inhibitor to date. 29 had a Morrison Ki of 63 pM and was about 40-fold more potent than 10 (Morrison Ki = 2.6 nM) and 250-fold more potent than 3a (Morrison Ki = 16 nM) in this G9a assay. UNC0321 potentially useful small molecule tools for the biomedical research community to investigate the biology of G9a and its role in chromatin remodeling as well as PTMs of other proteins. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 664.7±65.0 °C at 760 mmHg |
| Molecular Formula | C27H45N7O3 |
| Molecular Weight | 515.691 |
| Flash Point | 355.8±34.3 °C |
| Exact Mass | 515.358398 |
| PSA | 81.69000 |
| LogP | 0.36 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.582 |
| InChIKey | AULLUGALUBVBDD-UHFFFAOYSA-N |
| SMILES | COc1cc2c(NC3CCN(C)CC3)nc(N3CCCN(C)CC3)nc2cc1OCCOCCN(C)C |
| Storage condition | 2-8℃ |
|
~% 
UNC0321 CAS#:1238673-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 53, # 15 p. 5844 - 5857 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| 7-(2-(2-(Dimethylamino)ethoxy)ethoxy)-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine |
| 7-{2-[2-(Dimethylamino)ethoxy]ethoxy}-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methyl-4-piperidinyl)-4-quinazolinamine |
| UNC0321 |
| TFA salt |
![2-[2-(Dimethylamino)ethoxy]ethanol structure](https://image.chemsrc.com/caspic/385/1704-62-7.png)