Ajugamarin L2
Modify Date: 2025-08-21 17:04:31
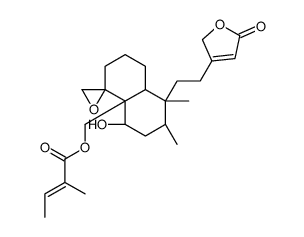
Ajugamarin L2 structure
|
Common Name | Ajugamarin L2 | ||
|---|---|---|---|---|
| CAS Number | 124961-67-7 | Molecular Weight | 432.55000 | |
| Density | 1.19g/cm3 | Boiling Point | 592ºC at 760 mmHg | |
| Molecular Formula | C25H36O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 196.5ºC | |
Use of Ajugamarin L2Ajudecunoid A is nature product that could be isolated from Ajuga decumbens. Ajudecunoid A inhibits RANKL-Induced osteoclastogenesis[1]. |
| Name | [(4aR,5S,7R,8S,8aR)-5-hydroxy-7,8-dimethyl-8-[2-(5-oxo-2H-furan-3-yl)ethyl]spiro[2,3,5,6,7,8a-hexahydro-1H-naphthalene-4,2'-oxirane]-4a-yl]methyl (E)-2-methylbut-2-enoate |
|---|---|
| Synonym | More Synonyms |
| Description | Ajudecunoid A is nature product that could be isolated from Ajuga decumbens. Ajudecunoid A inhibits RANKL-Induced osteoclastogenesis[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Ajudecunoid A (compound 1; 3 and 10 μM; 48 h) 以浓度依赖性方式抑制 RANKL 诱导的破骨细胞生成[1]。 Cell Viability Assay[1] Cell Line: Osteoclastogenesis Concentration: 3 and 10 Μm Incubation Time: 48 hours Result: Inhibited RANKL-induced osteoclastogenesis in a concentration dependent manner. |
| References |
| Density | 1.19g/cm3 |
|---|---|
| Boiling Point | 592ºC at 760 mmHg |
| Molecular Formula | C25H36O6 |
| Molecular Weight | 432.55000 |
| Flash Point | 196.5ºC |
| Exact Mass | 432.25100 |
| PSA | 85.36000 |
| LogP | 3.72170 |
| Vapour Pressure | 1.76E-16mmHg at 25°C |
| Index of Refraction | 1.553 |
| InChIKey | OPIBUIPAAPHGEN-ZRBKXSIYSA-N |
| SMILES | CC=C(C)C(=O)OCC12C(O)CC(C)C(C)(CCC3=CC(=O)OC3)C1CCCC21CO1 |
| Water Solubility | Very slightly soluble (0.48 g/L) (25 ºC) |
| Ajugacumbin B |
| Ajugamarin L2 |

