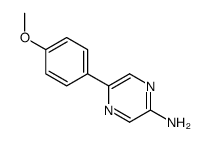
MCLA hydrochloride
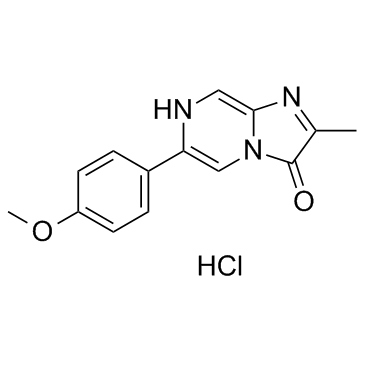
MCLA hydrochloride structure
|
Common Name | MCLA hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 128322-44-1 | Molecular Weight | 291.733 | |
| Density | N/A | Boiling Point | 410.3ºC at 760 mmHg | |
| Molecular Formula | C14H14ClN3O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 201.9ºC | |
Use of MCLA hydrochlorideMCLA hydrochloride is a chemiluminescent reagent which can be used to quantify aqueous concentrations of superoxide. |
| Name | 2-Methyl-6-(4-Methoxyphenyl)-3,7-Dihydroimidazo[1,2-a]Pyrazin-3-One Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | MCLA hydrochloride is a chemiluminescent reagent which can be used to quantify aqueous concentrations of superoxide. |
|---|---|
| Related Catalog | |
| In Vitro | The non-specific luminescence remains almost constant for 10 min after the addition of MCLA hydrochloride (MCLA) and is not significantly influenced by SOD. The MCLA hydrochloride method is, however, 4.5-times more sensitive than the CLA method[1]. |
| Kinase Assay | The standard reaction mixture contains 1×l0-7 M MCLA hydrochloride (MCLA), 5×10-5 M hypoxanthine, XOD (6.5 U), SOD (0.2 to 20 ng/mL) or none, and 50 mM Tris-HCl buffer containing 0.1 mM EDTA at pH 7.8, in a total volume of 3.0 mL. Chemiluminescence measurement is initiated by the addition of MCLA hydrochloride to the standard incubation mixture excluding XOD, continued for 4 min without additive and for an additional 4 min after the addition of XOD. Chemiluminescence is measured using a luminescence reader at 25°C[1]. |
| References |
| Boiling Point | 410.3ºC at 760 mmHg |
|---|---|
| Molecular Formula | C14H14ClN3O2 |
| Molecular Weight | 291.733 |
| Flash Point | 201.9ºC |
| Exact Mass | 291.077454 |
| PSA | 59.39000 |
| LogP | 2.80860 |
| Vapour Pressure | 3.95E-07mmHg at 25°C |
| InChIKey | MXZACTZQSGYANA-UHFFFAOYSA-N |
| SMILES | COc1ccc(-c2cn3c(O)c(C)nc3cn2)cc1.Cl |
| Storage condition | 2~8°C |
|
~62% 
MCLA hydrochloride CAS#:128322-44-1 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 9 p. 2475 - 2479 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Endothelial Angiogenesis and Barrier Function in Response to Thrombin Require Ca2+ Influx through the Na+/Ca2+ Exchanger.
J. Biol. Chem. 290 , 18412-28, (2015) Thrombin acts on the endothelium by activating protease-activated receptors (PARs). The endothelial thrombin-PAR system becomes deregulated during pathological conditions resulting in loss of barrier ... |
|
|
A sensitive and specific chemiluminescence method for estimating the ability of human granulocytes and monocytes to generate O2-.
Clin. Chim. Acta 179 , 177, (1989)
|
|
|
The first observation of O2- generation in in situ lungs of rats treated with drugs to induce experimental acute respiratory distress syndrome.
FEBS Lett. 261 , 369, (1990) To investigate O2- generation in in situ lungs of rats treated with drugs to induce experimental acute respiratory distress syndrome, phorbol myristate acetate (PMA) or endotoxin were injected into ra... |
| 2-Methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-one Hydrochloride |
| 6-(4-Methoxyphenyl)-2-methyl-3,7-dihydroimidazo[1,2-a]pyrazin-3(7H)-one hydrochloride |
| 6-(4-methoxyphenyl)-2-methyl-7H-imidazo[1,2-a]pyrazin-3-one,hydrochloride |
| MFCD00144728 |
| 6-(4-Methoxyphenyl)-2-methylimidazo[1,2-a]pyrazin-3(7H)-one hydrochloride (1:1) |
| Imidazo[1,2-a]pyrazin-3(7H)-one, 6-(4-methoxyphenyl)-2-methyl-, hydrochloride (1:1) |
| 6-(4-Methoxyphenyl)-2-methyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one Hydrochloride |
| 6-(4-Methoxyphenyl)-2-methylimidazo[1,2-a]pyrazin-3(7H)-one hydrochloride |
| MCLA hydrochloride |