Deserpidine
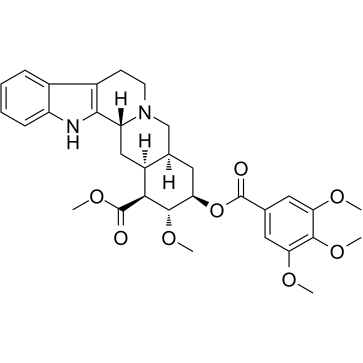
Deserpidine structure
|
Common Name | Deserpidine | ||
|---|---|---|---|---|
| CAS Number | 131-01-1 | Molecular Weight | 578.653 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 676.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C32H38N2O8 | Melting Point | ~275 °C (dec.)(lit.) | |
| MSDS | N/A | Flash Point | 362.7±31.5 °C | |
Use of DeserpidineDeserpidine (Harmonyl) is an alkaloid isolated from the root of Rauwolfia canescens related to Reserpine. Deserpidine is used as an antihypertensive agent and a tranquilizer. Deserpidine is a competitive angiotensin converting enzyme (ACE) inhibitor. Deserpidine also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex[1][2][3]. |
| Name | deserpidine |
|---|---|
| Synonym | More Synonyms |
| Description | Deserpidine (Harmonyl) is an alkaloid isolated from the root of Rauwolfia canescens related to Reserpine. Deserpidine is used as an antihypertensive agent and a tranquilizer. Deserpidine is a competitive angiotensin converting enzyme (ACE) inhibitor. Deserpidine also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Angiotensin converting enzyme (ACE)[3] |
| In Vitro | Deserpidine is an effective ganglionic blocking agent, which differs from Reserpine only by the absence of a methoxy group at C-11. Deserpidine has been used in the treatment of hypertension and psychosis. In addition, Deserpidine appears to act as a controller of other cardiac disorders[1][2]. |
| References |
[1]. Varchi G, et al. Synthesis of deserpidine from reserpine. J Nat Prod. 2005 Nov;68(11):1629-31. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 676.1±55.0 °C at 760 mmHg |
| Melting Point | ~275 °C (dec.)(lit.) |
| Molecular Formula | C32H38N2O8 |
| Molecular Weight | 578.653 |
| Flash Point | 362.7±31.5 °C |
| Exact Mass | 578.262817 |
| PSA | 108.55000 |
| LogP | 4.19 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.626 |
| InChIKey | CVBMAZKKCSYWQR-WCGOZPBSSA-N |
| SMILES | COC(=O)C1C2CC3c4[nH]c5ccccc5c4CCN3CC2CC(OC(=O)c2cc(OC)c(OC)c(OC)c2)C1OC |
| Storage condition | -20°C |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 9111 |
|---|---|
| WGK Germany | 3 |
|
~90% 
Deserpidine CAS#:131-01-1 |
| Literature: INDENA S.P.A. Patent: WO2005/95394 A1, 2005 ; Location in patent: Page/Page column 10-11 ; |
|
~% 
Deserpidine CAS#:131-01-1 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4084,4086 Journal of the American Chemical Society, , vol. 77, p. 4335,4340 |
|
~% 
Deserpidine CAS#:131-01-1 |
| Literature: Bulletin de la Societe Chimique de France, , p. 673,676 Annales Pharmaceutiques Francaises, , vol. 17, p. 15,21 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| methyl (3β,16β,17α,18β,20α)-17-methoxy-18-{[(3,4,5-trimethoxyphenyl)carbonyl]oxy}yohimban-16-carboxylate |
| DESERPIDINE |
| canescine |
| (3b,16b,17a,18b,20a)-17-Methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-3,20-yohimban-16-carboxylic Acid Methyl Ester |
| MFCD00078228 |
| Harmonyl |
| Recanescin |
| Canescin |
| Deserpidin |
| Methyl-(1S,2R,3R,4aS,13bR,14aS)-2-methoxy-3-{[(3,4,5-trimethoxyphenyl)carbonyl]oxy}-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2',3':3,4]pyrido[1,2-b]isochinolin-1-carboxylat |
| (1S,2R,3R,4aS,13bR,14aS)-2-méthoxy-3-{[(3,4,5-triméthoxyphényl)carbonyl]oxy}-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodécahydroindolo[2',3':3,4]pyrido[1,2-b]isoquinoléine-1-carboxylate de méthyle |
| Yohimban-16-carboxylic acid, 17-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-, methyl ester, (3b,16b,17a,18b,20a)- |
| Desepridine |
| 11-desmethoxyreserpine |
| 17a-Methoxy-18b-[(3,4,5-trimethoxybenzoyl)oxy]-3b,20a-yohimban-16b-carboxylic Acid Methyl Ester |
| 17-methoxy-18-(3,4,5-trimethoxy-benzoyloxy)-yohimbane-16-carboxylic acid methyl ester |
| Raunormin |
| Methyl (3β,16β,17α,18β,20α)-17-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]yohimban-16-carboxylate |
| 17α-Methoxy-18β-[(3,4,5-trimethoxybenzoyl)oxy]-3β,20α-yohimban-16β-carboxylic acid methyl ester |
| Yohimban-16-carboxylic acid, 17-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-, methyl ester, (3β,16β,17α,18β,20α)- |
| methyl (1R,15S,17R,18R,19S,20S)-18-methoxy-17-(3,4,5-trimethoxybenzoyl)oxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate |
| EINECS 205-004-8 |
| recanescine |
| Raunormine |
| Deserpidic Acid Methyl Ester 3,4,5-Trimethoxybenzoate |
| methyl (1S,2R,3R,4aS,13bR,14aS)-2-methoxy-3-{[(3,4,5-trimethoxyphenyl)carbonyl]oxy}-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2',3':3,4]pyrido[1,2-b]isoquinoline-1-carboxylate |