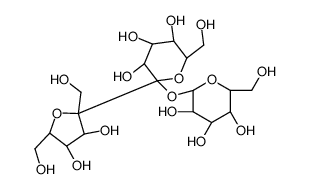
Erlose
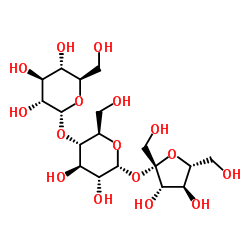
Erlose structure
|
Common Name | Erlose | ||
|---|---|---|---|---|
| CAS Number | 13101-54-7 | Molecular Weight | 504.437 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 889.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C18H32O16 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 491.6±34.3 °C | |
Use of ErloseErlose, a trisaccharide consisting of sucrose in soybean aphid honeydew, is utilized as a substitute sweetener preventing dental caries caused by oral flora, mainly Streptococcus mutans. Erlose may be used as a reference compound in HPLC assays that analyze the sugars of foods[1][2]. |
| Name | erlose |
|---|---|
| Synonym | More Synonyms |
| Description | Erlose, a trisaccharide consisting of sucrose in soybean aphid honeydew, is utilized as a substitute sweetener preventing dental caries caused by oral flora, mainly Streptococcus mutans. Erlose may be used as a reference compound in HPLC assays that analyze the sugars of foods[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 889.2±65.0 °C at 760 mmHg |
| Molecular Formula | C18H32O16 |
| Molecular Weight | 504.437 |
| Flash Point | 491.6±34.3 °C |
| Exact Mass | 504.169037 |
| PSA | 268.68000 |
| LogP | -2.85 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.676 |
| InChIKey | FVVCFHXLWDDRHG-KKNDGLDKSA-N |
| SMILES | OCC1OC(OC2C(CO)OC(OC3(CO)OC(CO)C(O)C3O)C(O)C2O)C(O)C(O)C1O |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 
Erlose CAS#:13101-54-7 |
| Literature: Glycobiology, , vol. 23, # 9 p. 1084 - 1096 |
|
~54% 
Erlose CAS#:13101-54-7 |
| Literature: Fujita; Kuwahara; Tanimoto; Koizumi; Iizuka; Minamiura; Furuichi; Kitahata Bioscience, Biotechnology and Biochemistry, 1994 , vol. 58, # 2 p. 239 - 243 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Simple column-switching ion chromatography method for determining eight monosaccharides and oligosaccharides in honeydew and nectar.
Food Chem. 194 , 555-60, (2015) Honeydew is excreted by aphids as a sweet waste and nectar is floral honey. Honeydew and nectar are complicated samples which consist of various sugars and amino acids. In this work, a simple ion chro... |
|
|
Sugar composition of French royal jelly for comparison with commercial and artificial sugar samples.
Food Chem. 134(2) , 1025-9, (2012) A gas chromatographic method was developed to quantify the major and minor sugars of 400 Royal Jellies (RJs). Their contents were compared in relation to the geographical origins and different product... |
|
|
NMR characterization of saccharides in Italian honeys of different floral sources.
J. Agric. Food Chem. 60(18) , 4526-34, (2012) The saccharide profiles of 5 different botanical species in 86 Italian honey samples were investigated by ¹H and ¹H-¹³C NMR spectroscopy. Nineteen saccharides were identified in the aqueous extracts, ... |
| α-D-Glucopyranoside, β-D-fructofuranosyl O-α-D-glucopyranosyl-(1->4)- |
| Erlose |
| β-D-Fructofuranosyl α-D-glucopyranosyl-(1->4)-α-D-glucopyranoside |
| α-Maltosyl β-D-fructofuranoside |