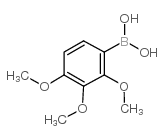
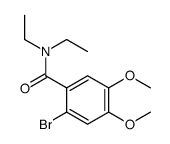
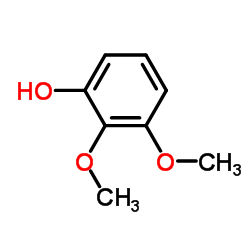
Urolithin D
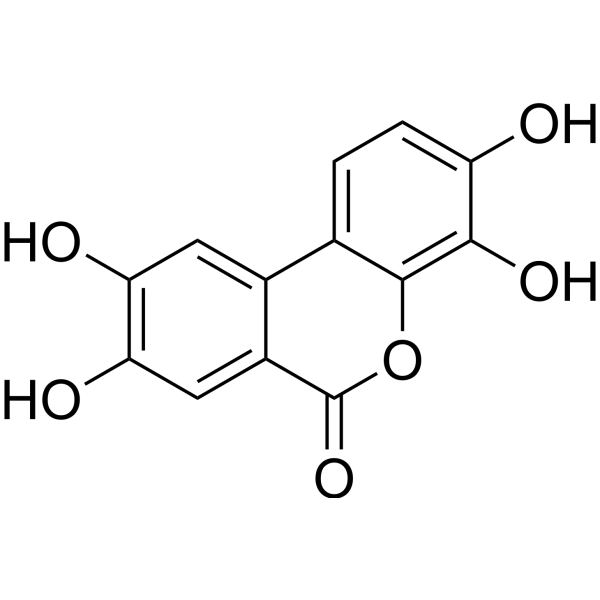
Urolithin D structure
|
Common Name | Urolithin D | ||
|---|---|---|---|---|
| CAS Number | 131086-98-1 | Molecular Weight | 260.20 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 634.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C13H8O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 253.0±25.0 °C | |
Use of Urolithin DUrolithin D is competitive and reversible antagonist of EphA receptors. Urolithin D exhibits intra-classes selectivity[1]. |
| Name | 3,4,8,9-tetrahydroxybenzo[c]chromen-6-one |
|---|---|
| Synonym | More Synonyms |
| Description | Urolithin D is competitive and reversible antagonist of EphA receptors. Urolithin D exhibits intra-classes selectivity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 634.1±55.0 °C at 760 mmHg |
| Molecular Formula | C13H8O6 |
| Molecular Weight | 260.20 |
| Flash Point | 253.0±25.0 °C |
| Exact Mass | 260.032074 |
| PSA | 111.13000 |
| LogP | 1.07 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.801 |
| InChIKey | NEZDQSKPNPRYAW-UHFFFAOYSA-N |
| SMILES | O=c1oc2c(O)c(O)ccc2c2cc(O)c(O)cc12 |
|
~% 
Urolithin D CAS#:131086-98-1 |
| Literature: AMAZENTIS SA; RINSCH, Christopher, L.; BLANCO-BOSE, William; SCHNEIDER, Bernard; MOUCHIROUD, Laurent; RYU, Dongryeol; ANDREUX, Penelope; AUWERX, Johan Patent: WO2014/4902 A2, 2014 ; Location in patent: Page/Page column 226-227 ; |
|
~% 
Urolithin D CAS#:131086-98-1 |
| Literature: Alo, B. I.; Kandil, A.; Patil, P. A.; Sharp, M. J.; Siddiqui, M. A.; et al. Journal of Organic Chemistry, 1991 , vol. 56, # 12 p. 3763 - 3768 |
|
~% 
Urolithin D CAS#:131086-98-1 |
| Literature: Alo, B. I.; Kandil, A.; Patil, P. A.; Sharp, M. J.; Siddiqui, M. A.; et al. Journal of Organic Chemistry, 1991 , vol. 56, # 12 p. 3763 - 3768 |
|
~% 
Urolithin D CAS#:131086-98-1 |
| Literature: AMAZENTIS SA; RINSCH, Christopher, L.; BLANCO-BOSE, William; SCHNEIDER, Bernard; MOUCHIROUD, Laurent; RYU, Dongryeol; ANDREUX, Penelope; AUWERX, Johan Patent: WO2014/4902 A2, 2014 ; |
|
~% 
Urolithin D CAS#:131086-98-1 |
| Literature: AMAZENTIS SA; RINSCH, Christopher, L.; BLANCO-BOSE, William; SCHNEIDER, Bernard; MOUCHIROUD, Laurent; RYU, Dongryeol; ANDREUX, Penelope; AUWERX, Johan Patent: WO2014/4902 A2, 2014 ; |
| 6H-Dibenzo[b,d]pyran-6-one, 3,4,8,9-tetrahydroxy- |
| 3,4,8,9-Tetrahydroxy-6H-benzo[c]chromen-6-one |
| 3,4,8,9-Tetrahydroxybenzo[c]chromen-6-one |
| 6H-Dibenzo(b,d)pyran-6-one, 3,4,8,9-tetrahydroxy- |
| urolithin D |
![3,4,8,9-tetramethoxy-6H-benzo[c]chromen-6-one structure](https://image.chemsrc.com/caspic/247/146776-39-8.png)