Cilnidipine
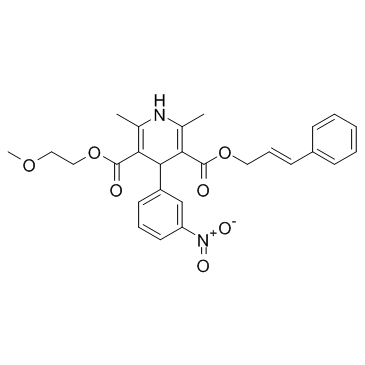
Cilnidipine structure
|
Common Name | Cilnidipine | ||
|---|---|---|---|---|
| CAS Number | 132203-70-4 | Molecular Weight | 492.520 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 652.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H28N2O7 | Melting Point | 97-99°C | |
| MSDS | Chinese USA | Flash Point | 348.5±31.5 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of CilnidipineCilnidipine(FRC8653) is a dual L- and N-type calcium channel blocker and displays antihypertensive, sympatholytic and neuroprotective activity. IC50 value:Target: calcium channelCilnidipine has displayed renal and vascular protective effects and improved baroreflex sensitivity in patients with hypertension. It has also demonstrated neuroprotective effects in a rat focal brain ischemia model by removing free radicals and activating the phosphatidylinositol 3-kinase pathway. |
| Name | cilnidipine |
|---|---|
| Synonym | More Synonyms |
| Description | Cilnidipine(FRC8653) is a dual L- and N-type calcium channel blocker and displays antihypertensive, sympatholytic and neuroprotective activity. IC50 value:Target: calcium channelCilnidipine has displayed renal and vascular protective effects and improved baroreflex sensitivity in patients with hypertension. It has also demonstrated neuroprotective effects in a rat focal brain ischemia model by removing free radicals and activating the phosphatidylinositol 3-kinase pathway. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 652.6±55.0 °C at 760 mmHg |
| Melting Point | 97-99°C |
| Molecular Formula | C27H28N2O7 |
| Molecular Weight | 492.520 |
| Flash Point | 348.5±31.5 °C |
| Exact Mass | 492.189636 |
| PSA | 119.68000 |
| LogP | 5.36 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.592 |
| InChIKey | KJEBULYHNRNJTE-DHZHZOJOSA-N |
| SMILES | COCCOC(=O)C1=C(C)NC(C)=C(C(=O)OCC=Cc2ccccc2)C1c1cccc([N+](=O)[O-])c1 |
| Storage condition | Store at +4°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R41 |
| Safety Phrases | S26;S39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | US7975550 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Preparation, characterization and tableting of cilnidipine solid dispersions.
Pak. J. Pharm. Sci. 26(3) , 629-36, (2013) Solid dispersion technique has been developed many years for improving solubility of water-insoluble drugs, aiming to achieve a better oral bioavailability. However, this technique exhibits many incon... |
|
|
The glucocorticoid mometasone furoate is a novel FXR ligand that decreases inflammatory but not metabolic gene expression.
Sci. Rep. 5 , 14086, (2015) The Farnesoid X receptor (FXR) regulates bile salt, glucose and cholesterol homeostasis by binding to DNA response elements, thereby activating gene expression (direct transactivation). FXR also inhib... |
|
|
Effects of cilnidipine on sympathetic outflow and sympathetic arterial pressure and heart rate regulations in rats.
Life Sci. 92(24-26) , 1202-7, (2013) Cilnidipine is a unique Ca(2+) channel blocker that inhibits both L-type and N-type Ca(2+) channels. The present study aimed to assess the effects of intravenous cilnidipine on sympathetic outflow and... |
| ASTA 3746 |
| 2-methoxyethyl (2E)-3-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate |
| Atelec |
| Ciclonium bromide |
| 2-Methoxyethyl (2E)-3-phenyl-2-propen-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate |
| Siscard |
| 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl (2E)-3-phenyl-2-propen-1-yl ester |
| MFCD00865853 |
| Cicloniumbromid |
| Cinalong |
| Cilnidipine |